States of Matter
All matter consists of atoms, and some atoms combine with others in a chemical reaction to form molecules. For example, water consists of two atoms of hydrogen combined with one atom of oxygen (H2O). Some gases such as argon (Ar), helium (He) and neon (Ne) are unlikely to combine with a similar or dissimilar atom, while others such as hydrogen, nitrogen, and oxygen and usually combine with an identical atom forming a molecule (H2, O2 and N2) and are called diatomic.
Matter is divided into three states: solid, liquid and gaseous. Some matter can exist in each of the three states, for example, water that can be ice (solid), water (liquid) and steam (vapor) depending on the surrounding pressure and temperature (Fig. 1). Another example is carbon dioxide that can also exist in all three states.
The Solid state
(Not to be confused with “solid state” electronic components)
In a solid, the atoms are bound tightly together in fixed positions relative to each other by interatomic forces. Therefore a solid has a fixed volume at a specific pressure and temperature. Changes in temperature will change the energy in the atoms and cause tiny vibrations about their position but the bonds are relatively strong and most solids can withstand reasonable temperature changes without breaking down apart from small expansion or contraction if the temperature changes. Even though a solid appears “solid” there are very small spaces between individual molecules. Metal solids, which are ones most prevalent in the vacuum furnace and heat treating industry, all have a melting point and if enough heat is applied the solid will eventually change to a liquid. This liquid, if more heat is added will eventually evaporate and change to a vapor.
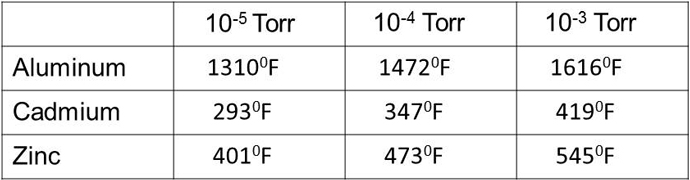
Vacuum chambers have to be made of selected metals that can withstand the heat of the process of course. They also have to be strong enough to withstand process pressures whether above or below the surrounding atmospheric pressure. One other requirement for vacuum chamber materials is that the material has a low vapor pressure, i.e. it does not outgas at process temperatures. The vapor pressure of most metals is very low and does not restrict their use. However, alloys that contain cadmium and zinc, for example, should be avoided when high temperature is a part of the process. Fig. 2 shows the saturated vapor pressure of cadmium and zinc, compared to aluminum, at three temperatures. Cadmium is used to plate some steel screws and brass contains zinc. Vapors evolved from hot metals will condense on any adjacent cooler surface. Not only will this contaminate the chamber but it may cause a shorting problem if the adjacent surface is an electrical insulator.
The Liquid state
In a liquid, the atoms and molecules have no fixed position and are in constant random motion in the bulk of the liquid. The atoms and molecules are still very close to each other and are held by interatomic forces. If heat is applied to a liquid the atoms and molecules will receive additional energy and start to move more vigorously. With enough energy, some atoms and molecules will overcome the binding forces and release from the liquid. This is the boiling point. At atmospheric pressure, i.e. boiling water on a stove, the liquid has to receive enough energy to overcome atmospheric pressure of around 14.7 lbsf in-2 before the water will boil or evaporate as steam.
When we consider high vacuum applications there are generally no liquids present in the vacuum chamber. The gaseous state and vapor molecules are much more of a concern.
The Gaseous state
In a gas, the atoms and molecules are generally much further apart than in solids and liquids. In air at atmospheric pressure and room temperature, the actual space occupied by atoms and molecules is about 0.01 percent or one ten-thousandth of the volume. The equivalent for solid copper is about 74 percent or close to three quarters. (So much for being called a “solid”).
In air the molecules are in constant random movement, typically in a straight line, and are the interatomic forces have little effect due to the space between the molecules. The moving molecules will constantly collide with other molecules and then move away in a different direction. These collisions occur about 10,000,000,000 times per second at atmospheric pressure.
In the atmosphere on earth, with wind and temperature changes, the air molecules will reside in areas that may have different densities of molecules. Then weather forecasters (meteorologists) talk about these areas as high pressure (denser) or low pressure (less dense) areas. High-pressure areas tend to deliver good weather and low-pressure areas are often bad news and bring rain or snow and high winds. Molecules will tend to move from higher pressure areas to low-pressure areas to equalize the pressure.
Inside a vacuum system at atmospheric pressure, before the pumps start the evacuation, the density of the gas molecules will be the same in all parts of the system. The density of the gas molecules changes as the chamber is evacuated, from relative high density at the start – atmospheric pressure, to a lower density under vacuum conditions.
In the initial roughing phase of a pumpdown, as the mechanical vacuum pumps remove molecules of gas from the vacuum piping the molecules in the main vacuum chamber move towards the lower pressure (lower density) at the vacuum pump inlet. The lower pressure is created by the mechanism of the vacuum pump as it draws in gas molecules and then expels them back into the atmosphere through the exhaust line. In time the pressure in the main chamber is reduced to that required for high vacuum pumping. At that point, the density of the molecules is so low and the distances between molecular collisions are so high that the gas doesn’t “flow” anymore. This is a discussion for another article.
Vapors and Saturated Vapor Pressure
If a dish of water is left open to atmosphere it will slowly evaporate, even at room temperature. Some molecules will have enough energy to release from the surface of the water and become part of the surrounding air. The surrounding air is generally called unsaturated, i.e. it can accept more vapor molecules.
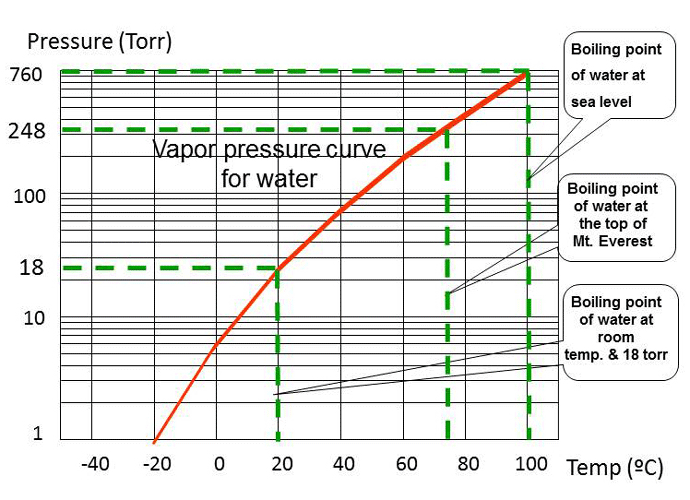
If that same dish of water was placed in a closed container, under a bell jar, for example, some molecules will still evaporate or leave the surface of the water and become part of the air inside the bell jar. At the same time, due to the enclosed volume, some molecules of water from the air will contact the surface of the water and condense back into the liquid. At some point, there will be as many molecules of water leaving the surface (evaporation) as there are joining the liquid (condensation) and the air volume is said to be saturated. The pressure the air exerts on the liquid is called the saturated vapor pressure. Since the rate of evaporation decreases with temperature – because the molecules have less energy, the saturated vapor pressure will also decrease. (Fig. 3)
Figure 3 shows the temperature required to evaporate (boil) water at several different pressures. The temperature needed to boil water is 100°C at 760 Torr (atmospheric pressure at sea level), 75°C at about 248 Torr (the top of Mount Everest) and 20°C (room temperature) at about 18 Torr (inside a vacuum chamber).
This article has introduced some basic information on states of matter and understanding how extremely small molecules of gas and vapor move around inside a vacuum system. This will help you visualize how the pumps operate and realize the difficulties involved in creating a low enough pressure to allow your process to be a success.
References:
Some material for this article was taken from “Modern Vacuum Practice” written and published by Nigel Harris, 3rd Revised Edition, Kurt J. Lesker Company, 2007. The values shown in Fig. 2 were converted from the original mbar and C degrees. Other material and comparisons are my own.
Copyright Howard Tring, Tring Enterprises LLC Vacuum & Low-Pressure Consulting.
