Abstract
Revealing the prior-austenite grain boundaries in heat treated steel is probably the most difficult, and frustrating task, faced by the metallographer or metallurgist. Grain boundaries, regardless of the type, are generally impossible to see in cast metals, as they solidify dendritically and segregation is present and often substantial. After deformation and annealing, if recrystallization occurs, grain boundaries in the product may be visible, but they are not necessarily prior-austenite grain boundaries. In a deformed, partially recrystallized specimen, it is usually possible to see both recrystallized and non-recrystallized grain boundaries. But, prior-austenite grain boundaries are those of the steel when it was austenitized prior to quenching and tempering. If the steel’s microstructure is fully martensitic after hardening, or contains some retained austenite or lower bainite, the prior-austenite grain boundaries may be revealed. They can often be revealed in specimens isothermally processed to obtain fully lower bainitic microstructures; but they cannot be revealed if the transformation microstructure consists of upper bainite, pearlite and/or ferrite. Composition also is important in trying to reveal the prior-austenite grain boundaries, as is the tempering temperature. In general, steels with low carbon contents and low phosphorous contents are very difficult subjects. This article summarizes the state-of-the-art in revealing prior-austenite grain boundaries.
Introduction
The size of the “parent” austenite grains of steels prior to quenching strongly influences the mechanical properties and service performance of heat treated alloy steels. Revealing the prior-austenite grain boundaries (PγGBs or PAGBs) is the most difficult task faced by ferrous metallographers. The degree of difficulty depends upon the alloy composition (C <0.3% and P <0.010% make it very difficult), tempering temperature (> 1060°F makes it very difficult) and its microstructure (only martensite, tempered martensite and lower bainite can be successfully etched). There are a number of well-established procedures [1] that are used to decorate the PγGBs during a heat treatment cycle, e.g., the McQuaid-Ehn carburizing test and the oxidation test. In some medium-carbon steels, at a specific cooling rate, proeutectoid ferrite will precipitate at the PγGBs while in high-carbon steels (generally hypereutectoid tool steels), proeutectoid cementite will precipitate on the PγGBs upon slow cooling from elevated temperatures. These conditions are often seen in as-cast or as-rolled steels. But, these methods cannot be applied to determine the prior-austenite grain size of a steel part or component that has already been heat treated as these methods will produce a different grain size. For this problem – and this is a common situation in failure analysis – one can only utilize an etching technique to reveal the PγGBs.
History of Prior-Austenite Grain Boundary Etch Development
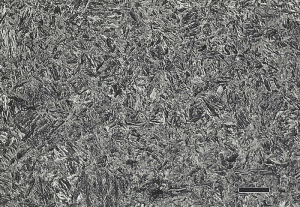
Vilella’s reagent (published in 1938) was one of the earliest etchants (1 g picric acid, 5 mL HCl, 100 mL ethanol) to have some limited success at revealing PγGBs in certain alloy steels [2], mainly with hot-work tool steels. In 1949, Miller and Day [3] published a 5% aqueous ferric chloride reagent for low-carbon martensitic steels. Aqueous ferric chloride and HCl solutions have also been suggested. Nital, general at a 10% concentration (do not store more than 3% HNO3 in ethanol in a tightly-closed bottle, as it can explode), will reveal the grain boundaries in only a few steels – highly alloyed tool steels in the as-quenched or lightly tempered condition, such as D2, D3 and high speed steels. Despite this, the writer has read comments on the internet by a metallographer claiming that he can reveal PγGBs with 2% nital on Q&T steels such as 4140. Figure 1 shows 4140 alloy steel austenitized at 1600°F and in the oil quenched condition plus after tempering at 400°F etched with 2% nital. There is absolutely no hint of any visible PγGBs, nor any grain contrast effects.
The first reasonably successful etchant for PγGBs was published in 1955 by Bechet and Beaujard [4] using a saturated aqueous picric acid solution (as had been used in studies of temper embrittlement) containing 0.5% of a wetting agent, “Teepol” (sodium alkyl sulfonate) by immersion at room temperature (Teepol is a registered trademark of the Shell Chemical Co, Houston, TX). This etchant has been the foundation of many subsequent modifications to improve its effectiveness.
The writer tried this etch [5] on specimens of 8620, 4140, and 5160 in the as-quenched condition and after tempering at 400, 800 and 1200°F using sodium tridecyl benzene sulfonate as the wetting agent (including the two specimens shown in Figure 1). It did not reveal grain boundaries on any of the 8620 specimens. It did reveal the PγGBs on as-quenched and tempered (400 and 800°F) specimens of 4140 and 5160, but did not reveal them on any specimens tempered at 1200°F. Tempered martensite and tempered bainite structures both respond to this etch, but only for medium to high-carbon steels, and only when tempered below ~1050°F. It is well known that saturated aqueous picric acid with a wetting agent (used at room temperature) reveals PγGBs if phosphorus is present in the grain boundaries and this is easier if the specimen has been heated in the temper embrittlement range [6]. Segregation of Sn or Sb to the PγGBs, which also cause temper embrittlement does not help reveal the PγGBs using this etch in steels free of phosphorus [7, 8]. Preece and Carter [9] showed using TEM that there was a clear difference in appearance between grain boundaries that were temper embrittled due to a high local phosphorous concentration compared to a non-embrittled specimen with a lower local phosphorous content, even though saturated aqueous picric acid revealed the PγGBs in both cases and the boundaries looked similar by light microscopy.
Studies conducted in this time period examined the effect of a variety of wetting agents on the etch response. Nelson [10] conducted the most extensive comparison using five wetting agents, including sodium tridecyl benzene sulfonate, with several different etchants including the saturated aqueous picric acid solution. Without the wetting agent added, saturated aqueous picric acid was an excellent general-purpose etchant for steels, but PγGBs were not revealed. When this wetting agent was added, general structure etching was suppressed and PγGBs were revealed. None of the other wetting agents tried were as effective. A number of studies on the use of wetting agents in etchants have been reviewed [11]. The original tridecyl version of this wetting agent has branched molecular chains which are difficult to manufacture and have poor biodegradability. More recent versions have linear chains and are biodegradable. Kilpatrick (1995, personal communication) evaluated the dodecyl version of this wetting agent, which is more easily made, is readily biodegradable, and works equally well. Consequently, this wetting agent is the most commonly used today for revealing PγGBs.
 |
 |
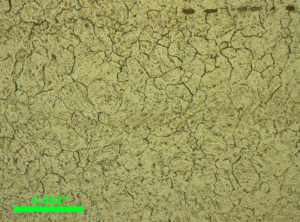 |
| a) Quenched and tempered 4123 alloy steel | b) Quenched and tempered 4130 alloy steel | c) Quenched and tempered 4140 alloy steel |
Krahe and Desnoues [12] tried bringing up PγGBs in high-purity Fe-C binary alloys using the aqueous saturated picric acid reagent but were unable to reveal them as this etch requires P segregation to the PγGBs. After a number of attempts with variations of this etchant, they obtained good results with a saturated ethanolic picric acid solution containing HCl and Teepol (100 cm3 ethanol, 10 cm3 of picric acid, 20 drops concentrated HCl and 20 drops of Teepol wetting agent). They found that the amount of HCl added was very important as too much caused pitting and too little resulted in failure to reveal the PγGBs. Their etchant, however, would not reveal PγGBs in Fe-C-Mn high-purity steels.
Barraclough [13] reviewed etchants tried by ten different authors to reveal PγGBs. He concluded that it was necessary to temper embrittle specimens to obtain adequate grain boundary delineation to permit measurements to be made of the grain size. His work confirmed that picric acid was the most suitable agent for revealing PγGBs and the solvent used was critical. Alcohols did not work, but water or ether gave good results (petroleum ether is less dangerous than ethyl ether, but both are explosive when heated above 100°C and static electricity can cause explosions). Several wetting agents were tried, all were suitable, but he preferred Teepol. He found that the aqueous solution could be used at temperatures up to 85°C, but did not indicate if higher temperatures produced any benefit or detriment. Barraclough used swabbing and lightly back-polished his specimens to reduce the etch details of the martensite within the grains; this is now an excellent common practice.
Brownrigg et al. [14] followed up on this study with a slight modification which they stated allowed them to bring out PγGBs for as-quenched steels from 0.03 to 0.8% C with bainitic structures. They used a solution of 100 mL saturated aqueous picric acid, plus 2 mL “Teepol” plus 6 drops of HCl. After mixing, they filtered out the excess picric acid which they stated reduced staining of the specimen surface. They immersed specimens at room temperature for 4 to 10 minutes. They demonstrated that PγGBs could be revealed in low-carbon (0.04%) as-rolled bainitic structures that were not recrystallized after hot rolling.
Bodnar et al. [15] studied development of PγGBs in CrMoV rotor steels using thirteen different etchants. The saturated aqueous picric acid etchant produced better results than most, but was still inadequate. Tempering specimens in the embrittlement range did not help as the phosphorous content was too low. Addition of a small amount (3-5 drops per 50 mL of etchant) of HCl to the etchant produced markedly better results. They etched for 5-8 minutes with the beaker placed in an ultrasonic cleaner for agitation (the water level in the ultrasonic cleaner must be lower than the etchant level in the beaker, or the beaker will flip over). This was followed by light re-polishing to remove some of the etch detail within the grains. Other etchants for revealing PγGBs have been developed; reference [5] lists 28 reagents published prior to 1984 for this purpose. The writer has previously published [16] a number of examples illustrating problems associated with revealing PAGBs in a variety of steels.
Experimental Procedure
Before specimens can be etched, they must be properly prepared to a very high quality level. The first and most critical step is sectioning which must be conducted to induce minimal damage. Use abrasive cut-off machines (avoid torch cutting, shearing, band saws or power hack saws as they induce far too much damage) with a blade/wheel designed for metallography and for steels of the hardness level being prepared. If the specimen you received was cut with one of these procedures, resection it with a proper abrasive cut off saw using the best blade for the alloys to be cut. Generally, mounting is performed, but may not be necessary if the structure at the edges of the sample is not important (as in a specimen cut from the interior of a part). Commence grinding with SiC paper, using as fine a grit size as possible. As a rule, start grinding with 120-grit SiC for steels ≥60 HRC; start with 180-grit SiC for steels between 35 and 60 HRC; and, start with 220-240-grit SiC for steels <35 HRC.
Next, polish the specimens using low resilience, non-nap cloths, such as DP/MD Plan or DP/MD-Pan with 9-µm diamond, using a load of 25-30 N per specimen, 150 rpm, for at least 5 minutes. Next, polish with DP/MD DAC or DP/MD DUR with 3-µm diamond, same load and rpm, for 5 minutes. For martensitic and bainitic steels that are to be etched to reveal prior-austenite grain boundaries, a 1-µm step is not necessary and the final step would be using either a synthetic neoprene cloth, such as DP/MD-Chem, or a napped or flocked cloth, such as DP/MD-Floc or DP/MD-Nap using either colloidal silica, such as OP-S or a neutral alumina suspension, such as OP-AN. I prefer to use “contra” rotation (head in the opposite direction of the platen with the head rotating at 60 rpm) for all steps after grinding. Polishing is usually conducted at 120-150 rpm, same load, from 1 to 3 minutes. A good practice is to lightly etch the specimens after the last step with a general-purpose reagent, such as 2% nital, to see what the structure actually is and how well prepared the specimens are before proceeding to use the saturated aqueous picric acid etch. After examination, repeat the last step for at least 1 minute to remove this etch. Cleaning after each step is important to prevent contamination of the next step and poor results.
The writer has been using saturated aqueous picric acid plus a wetting agent and a small HCl addition (when steels have more than about 1% Cr) for some time, but formerly at room temperature. The specimen would be placed polished face vertical in a beaker with at least 100 mL of the etchant in an ultrasonic cleaner (the water level in the ultrasonic cleaner should not be higher than the etchant level in the beaker or it will flip over). The timer would be set for 7 minutes with etching at room temperature. Results with 8620, 4140 and 5160 were described above (without adding HCl). Light back-polishing was always done to try and improved the visibility of the grain boundaries. As sodium tridecyl benzene sulfonate became difficult to obtain, the writer switched to the dodecyl version with no apparent difference.
 |
||
| 1600°F | 1700°F | 1800°F |
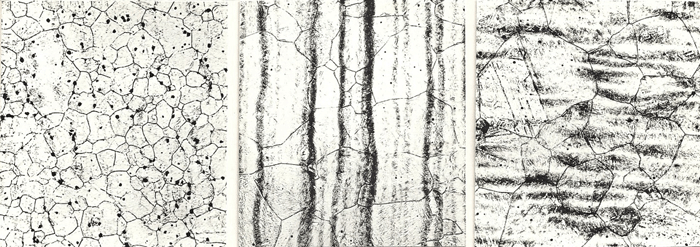 |
||
| 1900°F | 2000°F | 2100°F |
 |
||
| 2200°F | 2300°F | 2400°F |
| Figure 3: Prior-austenite grain boundaries revealed in 4340 alloy steel specimens austenitized from 1600 to 2400°F, oil quenched and tempered at 400°F using saturated aqueous picric acid, 1% HCl and sodium dodecyl benzene sulfonate wetting agent. Note the progressive increase in grain size with increasing austenitizing temperature. Images at 100X. |
Results
The first experimental results illustrate the effect of carbon content on the ability to reveal the prior-austenite grain boundaries. A series of Cr-Mo 41XX alloy steels with carbon contents from ~0.2 to 0.8% were austenitized at the property temperature for each grade, oil quenched and lightly tempered. They were etched with saturated aqueous picric acid with a small HCl addition, ~0.6%) and with sodium dodecyl benzene sulfonate as the wetting agent at 80-90°C. The prior-austenite grain sizes varied from ASTM 9.6 to 10.3. The 4123 and 4130 specimens were more difficult to etch than the 4140, 4160 and 4180 specimens. Figure 2 shows examples of the etch results.
The next example, shown in Figure 3, is a series of 4340 alloy steel specimens (Fe-0.41% C-0.74% Mn-0.018% P-0.015% S-0.31% Si-1.82% Ni-0.78% Cr-0.25% Mo-0.047% Al-0.0010% N) that were austenitized at temperatures from 1600 to 2400°F, oil quenched and lightly tempered. These were etched at room temperature with saturated aqueous picric acid, ~1% HCl and sodium dodecyl benzene sulfonate as the wetting agent. Due to the 0.018% phosphorous content, the 0.41% carbon content and the fully martensitic microstructure and low tempering temperature, the prior-austenite grain boundaries were relatively easy to reveal. The series shows a gradual grain growth with increasing austenizing temperature which increases above 1900°F after the aluminum nitride was put into solution.
Conclusions
Revealing prior-austenite grain boundaries has been one of the most difficult and most frustrating tasks assigned to the metallography/materialography laboratory. The most successful etch had been the saturated aqueous picric acid containing a wetting agent, usually sodium dodecyl benzene sulfonate, at room temperature for periods of 4-20 minutes. However, this etch was unable to reveal PγGBs in martensitic or bainitic steels with carbon contents below ~0.3%, or with phosphorus contents below ~0.010%, even when subjected to step-embrittlement cycles, or for steels tempered above ~1050°F.
However, if a small amount of HCl is added, usually 0.6 to 1%, and the etchant is used at ~80-90°C (results were good at 70°C when tried on one specimen), these limitations are overcome. Avoid using the etch when boiling as its composition will vary with time. Filtering the solution before use does help reduce staining/pitting attack. Careful, low-pressure back-polishing on a stationary cloth using OP-AN alumina slurry is very effective at reducing extraneous etch detail within the grains and enhancing grain boundary visibility.
References
- ASTM E 112-12, Standard Test Methods for Determining Average Grain Size,” Annex A3, “Austenite Grain Size, Ferritic and Austenitic Steels.”
- J.R. Vilella, Metallographic Techniques for Steel, American Society for Metals, Cleveland, Ohio, 1938.
- O.O. Miller and M.J. Day, “Ferric Chloride Etchant for Austenite Grain Size of Low-Carbon Steel,” Metal Progress, Vol. 56, 1949, pp. 692-695.
- S. Bechet and L. Beaujard, “New Reagent for the Micrographical Demonstration of the Austenite Grain of Hardened or Hardened-Tempered Steels,” Rev. Met., Vol. 52, 1955, pp. 830-836.
- G.F. Vander Voort, Metallography: Principles and Practice, McGraw-Hill Book Co., NY, 1984 and ASM International, Metals Park, OH, 1999, p. 222.
- A.H. Ücisik, H.C. Feng and C.J. McMahon, “The Influence of Intercritical Heat Treatment on the Temper Embrittlement of a P-Doped Ni-Cr Steel,” Metall. Trans., Vol. 9A, 1978, pp. 321-329.
- A.K. Cianelli et al., “Temper Embrittlement of a Ni-Cr Steel by Sn,” Metall. Trans., Vol. 8A, 1977, pp. 1059-1061.
- A.H. Ücisik, C.J. McMahon and H.C. Feng, “The Influence of Intercritical Heat treatment on the Temper Embrittlement Susceptibility of an Sb-doped Ni-Cr Steel,” Metall. Trans., Vol. 9A, 1978, pp. 604-606.
- A. Preece and R.D. Carter, “Temper-Brittleness in High-Purity Iron-Base Alloys,” J. Iron and Steel Inst., Vol. 173, 1953, pp. 387-398.
- J.A. Nelson, “The Use of Wetting Agents in Metallographic Etchants,” Praktische Metallographie, Vol. 4, 1967, pp. 192-198.
- G.F. Vander Voort, “Wetting Agents in Metallography,” Materials Characterization, Vol. 35, September 1995, pp. 135-137.
- P. R. Krahe and M. Desnoues, “Revealing the Former Austenite Grain Boundaries of High-Purity Iron-carbon Alloys,” Metallography, Vol. 4, 1971, pp. 171-175.
- D.R. Barraclough, “Etching of Prior Austenite Grain Boundaries in Martensite,” Metallography, Vol. 6, 1973, pp. 465-472.
- A. Brownrigg et al., “Etching of Prior Austenite Grain Boundaries in Martensite,” Metallography, Vol. 8, 1975, pp. 529-533.
- R.L. Bodnar et al., “Technique for Revealing Prior Austenite Grain Boundaries in CrMoV Turbine Rotor Steel,” Metallography, Vol. 17, 1984, pp. 109-114.
- G. F. Vander Voort, “Revealing Prior-Austenite Grain Boundaries in Heat-Treated Steels,” Industrial Heating, Vol. 78, April 2010, pp. 48-52.
George Vander Voort has a background in physical, process and mechanical metallurgy and has been performing metallographic studies for 47 years. He is a long-time member of ASTM Committee E-4 on metallography and has published extensively in metallography and failure analysis. He regularly teaches MEI courses for ASM International and is now doing webinars. He is a consultant for Struers Inc. and will be teaching courses soon for them. He can be reached at 1-847-623-7648, EMAIL: georgevandervoort@yahoo.com and through his web site: www.georgevandervoort.com
To View a listing of all George’s articles please click here
Read George Vander Voort’s Biography