Introduction
 The microstructure of iron-based alloys is very complicated, being influenced by composition, homogeneity, processing and section size. Microstructures in coarse-grained steels are much easier to observe than in fine-grained steels. Of course, steels are normally made with a fine grain size for best mechanical properties. In general, it is easiest to identify heat-treated structures after transformation and before tempering. But, in most applications, hardened steels must be tempered and they are usually examined in this condition. If a mixed microstructure of bainite and martensite is formed during quenching, these constituents will become more difficult to identify reliably as the tempering temperature given the product increases towards the lower critical temperature. These factors make it more difficult to identify phases and constituents in steels. Further, while ferrous metallographers tend to use nital almost exclusively for etching, nital is not always the best reagent to use to properly reveal all microstructures. It is unfortunate that some companies prohibit use of picral because picric acid can be made to detonate under certain conditions. Picral is an excellent etchant for revealing certain microstructural constituents in steel and accidents have been less common than for nital. In this paper, isothermally transformed microstructures will be examined as an aid to phase/constituent identification. Further, the value of a tint etchant, 10% sodium metabisulfite, for revealing steel phases and constituents will be demonstrated.
The microstructure of iron-based alloys is very complicated, being influenced by composition, homogeneity, processing and section size. Microstructures in coarse-grained steels are much easier to observe than in fine-grained steels. Of course, steels are normally made with a fine grain size for best mechanical properties. In general, it is easiest to identify heat-treated structures after transformation and before tempering. But, in most applications, hardened steels must be tempered and they are usually examined in this condition. If a mixed microstructure of bainite and martensite is formed during quenching, these constituents will become more difficult to identify reliably as the tempering temperature given the product increases towards the lower critical temperature. These factors make it more difficult to identify phases and constituents in steels. Further, while ferrous metallographers tend to use nital almost exclusively for etching, nital is not always the best reagent to use to properly reveal all microstructures. It is unfortunate that some companies prohibit use of picral because picric acid can be made to detonate under certain conditions. Picral is an excellent etchant for revealing certain microstructural constituents in steel and accidents have been less common than for nital. In this paper, isothermally transformed microstructures will be examined as an aid to phase/constituent identification. Further, the value of a tint etchant, 10% sodium metabisulfite, for revealing steel phases and constituents will be demonstrated.
TERMINOLOGY
There is confusion and misusage of certain microstructural terms. Sorbite and troostite were dropped from the metallographic lexicon in 1937 because they referred to microstructural constituents inaccurately. However, such terms are still occasionally used. The term “phase” is often used incorrectly in reference to mixtures of two phases, such as pearlite or bainite, which are more properly called constituents. A phase is a homogeneous, physically distinct substance. Martensite is a phase when formed by quenching but becomes a constituent after tempering as in decomposes from body-centered tetragonal (bct) martensite to body-centered cubic (bcc) ferrite and orthorhombic cementite.
SPECIMEN PREPARATION
In order to observe the microstructure of ferrous metals, they must be properly prepared. Many view this task as a trivial exercise, yet its proper execution is critical to successful interpretation. The first step is in selecting the test locations to be sampled. This is critical if the interpretation is to be valid for the part or lot being evaluated. The specimens selected must be representative of the lot. The plane of polish may be oriented in different directions relative to the piece being sampled.
Sectioning is almost always required to obtain a piece of the proper size and orientation for metallographic examination. The abrasive cut-off saw is the most commonly used device for sectioning. It produces good surfaces with minimal damage, when the proper blade is used with adequate coolant. More aggressive sectioning methods are often used in production operations. These produce greater damage to the structure that must be removed if the true structure is to be examined.
Next, the specimen may be mounted in a polymeric material to facilitate handling, to simplify preparation, to enhance edge retention, or for ease of identification of the specimen. Mounting may be done in a press using a thermosetting or thermoplastic resin or with castable resins that do not require external heat and pressure for polymerization.
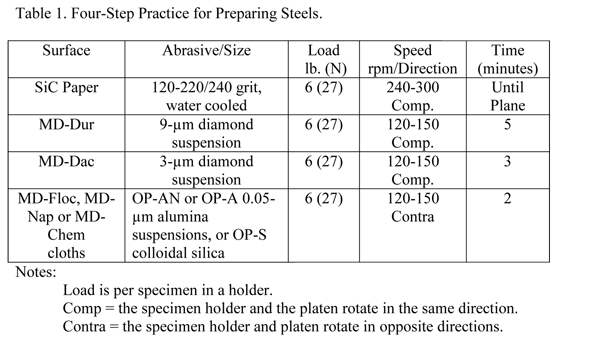
The use of automation in specimen preparation has grown enormously over the past twenty-five years. These devices produce better results than can be achieved manually. They yield more consistent results, better flatness and better edge retention, with greater productivity. Many procedures can be listed for preparing ferrous specimens successfully. There is no one correct procedure. Many different products can be used with successful results. Some methods may favor certain types of specimens or problems. Recognizing this situation, Table 1 lists a useful procedure for preparing most steel specimens. This method gives consistent results with good edge retention. For the most difficult specimens, a 1-µm diamond step can be added after the 3-µm diamond step, using the same materials, speeds and direction, but somewhat less time.
Notes:
- Load is per specimen in a holder.
- Comp = the specimen holder and the platen rotate in the same direction.
- Contra = the specimen holder and platen rotate in opposite directions.
Other variations are possible depending upon needs and specimens. The first step, often called planar grinding, can be done using several products. The traditional silicon carbide paper is always satisfactory; aluminum oxide paper may also be used. Always start with the finest possible abrasive that can remove the damage from cutting and get all of the specimens in the holder co-planar in a reasonable time. SiC paper does have a short life. Continuing to grind after the paper has lost its cutting efficiency will generate heat and damage.
ETCHANTS
Nital, usually a 2% concentration, is most commonly used to reveal the microstructure of steels. It is excellent for revealing the structure of martensite. Nital is also very good for revealing ferrite in a martensitic matrix and for ferrite grain boundaries in low-carbon steels. Picral reveals the cementite in ferritic alloys and the structure of ferrite-cementite constituents, pearlite and bainite, but does not reveal the ferrite grain boundaries. Nital and picral both dissolve ferrite but nital’s dissolution rate is a function of crystal orientation while picral’s rate is uniform. Other reagents have their uses, especially when dealing with higher alloy grades or when trying to selectively reveal certain constituents or prior-austenite grain boundaries. Etchants for steels are listed in many standard textbooks (1) and handbooks, and in ASTM E 407.
Because some laboratories cannot use picral, specimens were also etched with 10% sodium metabisulfite (also called sodium pyrosulfite) which has many of the virtues of both nital and picral. Add 10 g Na2S2O5 to 100 mL of water to make this etch. Sodium metabisulfite (SMB) is quite safe to use. Of course, it should not be ingested and direct contact should be avoided. Compared to other tint etchants, it is relatively simple to use. It will etch a wide range of compositions and does produce coloration, which is not strong, but can be enhanced using partially cross polars with a sensitive tint plate. It will lightly color some ferrite grains and will reveal ferrite grain boundaries, like nital, but with greater uniformity. It reveals pearlite and bainite as well as picral. It reveals as-quenched martensite as well as nital. Etching was done by immersion and times were typically 5-15 s. 10% SMB can be stored.
ISOTHERMAL TRANSFORMATIONS
Microstructures, even in steels, were not well understood and heat treatment was more of an art than a science until Davenport and Bain (2) published their landmark paper on the isothermal transformation of austenite in 1930. This led to maps of isothermal transformations using a time-temperature-transformation (TTT) diagram (3, 4) for each steel composition. These diagrams have been developed for many compositions using several approaches (5). Isothermally-transformed specimens are highly useful in teaching interpretation of microstructures. Austenite transformation can also be studied using controlled cooling rates and can be plotted in this way (CCT diagrams). In this paper, only isothermally transformed structures, except for martensite, will be illustrated (the untransformed austenite is converted to martensite during the quench after the isothermal hold).
The carbon content of an alloy is a major factor influencing the amount and appearance of phases and constituents. The specimens illustrated were austenitized at the normal recommended temperatures and then held isothermally at different sub-critical temperatures in a salt bath to convert some or all of the austenite to other phases and constituents.
MICROSTRUCTURES
Ferrite and Pearlite
Alpha iron, strictly speaking, refers only to the body-centered cubic (bcc) form of pure iron that is stable below 912 ºC (1674 ºF) while ferrite is a solid solution of one or more elements in bcc iron. Often these terms are used as synonyms, which is not correct. Ferrite may precipitate from austenite in acicular form (Widmanstätten ferrite) under certain cooling conditions. Sheet steels are fully ferritic and there are fully ferritic silicon electrical steels and stainless steels. Ferrite is a very soft, ductile phase, although it looses its toughness below some critical temperature.
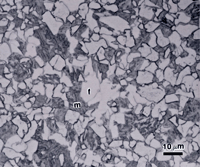
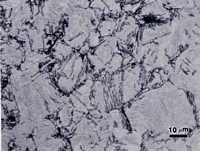
If the isothermal transformation temperature is close to the lower critical temperature, and the steel is hypoeutectoid (< 0.8% C), ferrite will precipitate first before pearlite is formed. This ferrite is called proeutectoid ferrite. The amount of proeutectoid ferrite decreases as the carbon content increases and as the isothermal transformation temperature is decreased towards the “nose” of the TTT diagram. Figure 1 shows proeutectoid ferrite and martensite in 8620 alloy steel (0.2%C-0.8%Mn-0.25% Si-0.55%Ni-0.5%Cr-0.2%Mo) austenitized at 927 ºC, isothermally transformed at 677 ºC for 1 minute, and then water quenched. The specimens were etched with 2% nital, 4% picral and 10% SMB. The holding time was short enough so that only ferrite formed isothermally before the specimen was water quenched (which transformed the remaining austenite to martensite). Figure 1 shows that nital revealed the martensite well and left the ferrite white while revealing ferrite-ferrite grain boundaries. 10% SMB revealed the martensite (m) in similar manner, slightly colored some of the ferrite (f) grains, and also revealed the ferrite grain boundaries. However, 4% picral revealed nothing (the slight relief between the ferrite and martensite, due to hardness differences can be seen, as the aperture diaphragm was stopped down).
After the proeutectoid ferrite is precipitated during an isothermal hold, such as illustrated in Figure 1, pearlite will form if the hold is continued. Pearlite is a metastable lamellar aggregate of ferrite and cementite that forms at temperatures below the lower critical temperature (the temperature where austenite starts to form upon heating). Pearlite forms by the eutectoid reaction where solid austenite transforms to solid ferrite and cementite with lamellar morphologies upon cooling from the austenite field. The reverse happens with heating into the austenite field.
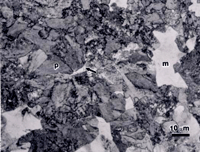
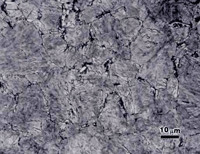
There is no separate region on the TTT diagram for a high carbon alloy steel, such as SAE 5160, indicating formation of proeutectoid ferrite, as the maximum content that can be isothermally formed is less than 1%. Figure 2 shows the microstructure of a specimen of SAE 5160 alloy steel (0.6%C-0.85%Mn-0.25%Si-0.8%Cr) that was austenitized at 830 ºC, isothermally transformed at 677 ºC for 30 seconds, and water quenched. For this 5160 specimen, we observe a few tiny patches of proeutectoid ferrite (much less than 1% by volume), pearlite and as-quenched martensite. The interlamellar spacing of the pearlite is too fine to resolve.
Each image in Figure 2 was selected to show at least one grain of proeutectoid ferrite (arrows). These are not typical fields, as most fields contained no ferrite. Nital is not entirely satisfactory for this microstructure. The large light patches appear to have some faint structure in them, but it is unclear if this is as-quenched martensite (m), ferrite, or lightly etched pearlite. The picral etch reveals uniformly etched pearlite (p) and large white patches that are as-quenched martensite (m). But, the white patches of unetched martensite could be mistaken for ferrite if one did not realize that this much ferrite could never be produced in such a high carbon alloy. It is also hard to determine if the small patches of proeutectoid ferrite are, or are not, as-quenched martensite based upon this etch. Viewed through the green filter in black and white, the 10% SMB etch reveals all of the constituents, and as-quenched martensite can be distinguished from fine pearlite, but this is not an easy distinction. Viewed in color, the distinction is quite clear because the martensite is colored brown while the fine pearlite has a multitude of colors (when viewed with partially crossed polars and a sensitive tint plate).
Bainite
If the isothermal transformation temperature is lowered to below the “nose” of the TTT curve, but not below the temperature where martensite starts to form (the Ms temperature), a different two-phase constituent may be observed, called bainite. Bainite is a metastable aggregate of ferrite and cementite that forms from austenite at temperatures below where pearlite forms and above the temperature where martensite starts to form. The appearance of bainite changes with the transformation temperature being called “feathery” in appearance at high temperatures and “acicular” at low transformation temperatures. The feathery appearance of “upper” bainite is also influenced by carbon content and is most appropriate for grades with high carbon contents. The acicular description is not a prefect description of the shape of “lower” bainite.
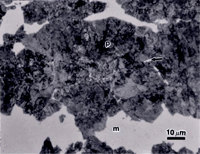
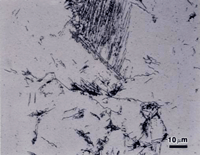
Figure 3 shows the microstructure of 5160 alloy steel austenitized at 830 ºC, isothermally transformed at 538 ºC for 60 seconds and water quenched which produces a small amount of upper bainite in a martensitic matrix (the austenite that did not transform). The structures are revealed using nital, picral and sodium metabisulfite. Picral does not reveal the martensitic matrix at all. (If the specimen was tempered, or if excessive heat was generated cutting the specimen, picral would bring up the martensite.) It is lightly developed by nital (over-etching would make it darker), and somewhat darker with SMB. Again, the SMB image reveals more in color (not shown). All three etchants reveal the structure of the upper bainite.
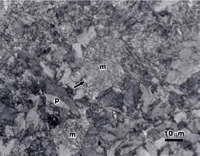
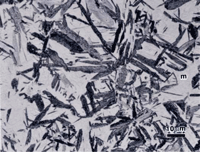
Figure 4 shows lower bainite and as-quenched martensite in alloy 5160. The specimen was austenized as before but isothermally transformed at 343 ºC for 5 minutes followed by water quenching. Again, all three etchants were used to reveal the two constituents. The same basic trends are observed. Sodium metabisulfite produced the strongest overall contrast and best visibility for both constituents. Picral is the most delicate and produces the greatest contrast difference between the unetched martensite and the lower bainite. The two-phase nature of bainite is best depicted by picral.
Martensite
If the cooling rate from the austenitizing temperature is rapid enough (a function of section size, hardenability and quench medium) so that the above-mentioned phases do not form, martensite will be produced. Of course, this is not an isothermal treatment, but is diffusionless. Martensite is a generic term for the body-centered tetragonal phase that forms by diffusionless transformation and the parent and product phases have the same composition and a specific crystallographic relationship. Martensite can be formed in alloys where the solute atoms occupy interstitial sites, as for C in Fe, producing substantial hardening and a highly stained, brittle condition. However, in carbon-free alloys with high nickel contents, such as maraging steels, the solute atoms (Ni) can occupy substitutional sites, producing martensites that are soft and ductile. In carbon-containing steels, the appearance of the martensite changes with carbon in the interstitial sites. Low carbon steels produce lath martensites while high carbon steels produce plate martensite, often incorrectly called “acicular” martensite, when all of the carbon is dissolved into the austenite. Martensite was shown in most of the micrographs where the transformation time was not great enough to convert all of the austenite to ferrite and pearlite or bainite.
SUMMARY
The microstructure of ferrous alloys is quite complicated. Identification of phases or constituents in steels depends upon proper specimen preparation and etching. The examples given showed that nital and picral do reveal the structure quite differently. 10% aqueous sodium metabisulfite behaves much like nital but is more uniform in its action. Unlike picral, it will reveal the structure of as-quenched martensite. It is excellent for revealing the diffusion-controlled products, ferrite, pearlite and bainite and the diffusionless product, martensite. It often gave the best contrast and is safer to use than nital or picral. .
REFERENCES
- G.F. Vander Voort, Metallography: Principles and Practice, ASM International, Materials Park, OH, 1999.
- E.S. Davenport and E.C. Bain, “Transformation of Austenite at Constant Subcritical Temperatures,” Trans. AIME, 1930, p. 117.
- E.S. Davenport, “Isothermal Transformation in Steels,” Trans. ASM, Vol. 27, 1939, p. 837.
- Isothermal Transformation Diagrams, 3rd ed., United States Steel, Pittsburgh, PA, 1963.
- G.F. Vander Voort, Atlas of Time-Temperature Diagrams for Irons and Steels, ASM International, Materials Park, OH, 1991.
George Vander Voorthas a background in physical, process and mechanical metallurgy and has been performing metallographic studies for 43 years. He is a long-time member of ASTM Committee E-4 on metallography and has published extensively in metallography and failure analysis. He regularly teaches MEI courses for ASM International and is now doing webinars. He is a consultant for Struers Inc. and will be teaching courses soon for them. He can be reached at 1-847-623-7648, EMAIL: georgevandervoort (AT) yahoo (DOT) com and through his web site: www.georgevandervoort.com
To View a listing of all George’s articles please click here
Read George Vander Voort’s Biography