A number of etchants have been reported in the literature to selectively outline, outline and color, or attack specific types of carbides in steels. These etchants have been developed during the first half of the 20th century but have not been studied systematically since the development of modern analytical techniques. To evaluate these etchants, eight specimens (seven different compositions) with M3C, M23C6, M7C3, M6C, M2C and MC carbides were studied, first by electron-backscattered diffraction (EBSD) to verify the carbides present. The matrix of each specimen was darkened to measure the total carbide content. Then, the various etchants were tried and the results were compared to past publication results. Quantitative measurements were made after each etchant was used. This revealed some minor differences with the prior literature and showed that, while useful for qualitative evaluations, they are not useful for quantitative measurements.
 Metallographers have used certain selective etchants for years to color specific carbides as an aid to phase identification. However, only a few of these etchants have had wide usage (e.g., alkaline sodium picrate to color cementite since 1906 due to the work by Kourbatoff). The behaviors of the others are less well known. Even for alkaline sodium picrate, however, few metallographers know that it also outlines and colors M6C carbides.
Metallographers have used certain selective etchants for years to color specific carbides as an aid to phase identification. However, only a few of these etchants have had wide usage (e.g., alkaline sodium picrate to color cementite since 1906 due to the work by Kourbatoff). The behaviors of the others are less well known. Even for alkaline sodium picrate, however, few metallographers know that it also outlines and colors M6C carbides.
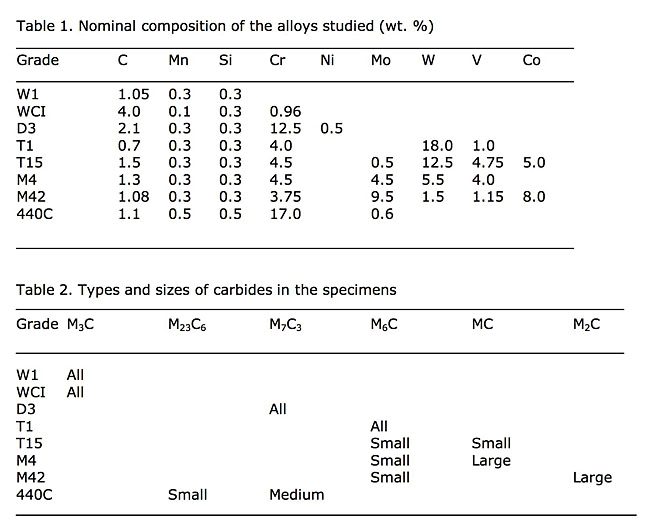
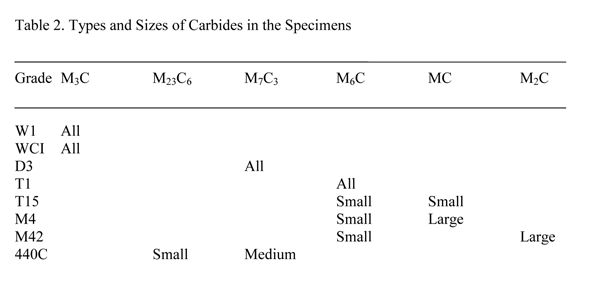
The nominal compositions of the alloys used in this study are given in Table 1. For the white cast iron (WCI) specimen, Table 1 gives the actual composition. The 440C martensitic stainless steel, the M42 and the T15 high-speed steel specimens are all annealed powder-metallurgy alloys with fine carbide sizes. The other tool steels were made by ingot technology. The D3 specimen was austenitized at 1066ºC, which is higher than the recommended temperature (1010ºC) to dissolve all of the small carbides, then quenched to form martensite. The W1 and the M4 specimens were spheroidize annealed. Both spheroidize annealed and quenched and tempered specimens of M42 high-speed steel were evaluated. The white cast iron specimen was, of course, in the as-cast condition. The specimens were prepared and analyzed using EBSD on a focused ion-beam unit by Dr. J. R. Michael. The results are given in Table 2.
 A variety of etchants were tried to darken the matrix. Klemm’s I gave excellent results for the spheroidize-annealed W1 and the white cast iron (cementite and pearlite as Ledeburite) specimens. A Beraha-type solution (100 mL water, 0.6 mL HCl, 1 g K2S2O5) was used to color the ferritic matrix of annealed T15 and M42 and to color the martensitic matrix in the M42 specimens. Vilella’s reagent was used to darken the martensite in the D3 specimen. Beraha’s Sulfamic acid reagent (100 mL water, 3 g K2S2O5, 2 g Sulfamic acid and 1 g NH4·FHF) was used to color the ferritic annealed matrix of the T1 and M42 specimens. Other reagents were tried, but these yielded the best contrast between the unaffected carbides and the colored matrix while yielding crisp grain boundaries. The specimens were evaluated by automated image analysis to determine the volume fraction, number per unit area and size of the carbides. Next, the specimens were re-prepared and etched with the reagents listed in Table 3. Results of the etching experiments are shown in Table 4.
A variety of etchants were tried to darken the matrix. Klemm’s I gave excellent results for the spheroidize-annealed W1 and the white cast iron (cementite and pearlite as Ledeburite) specimens. A Beraha-type solution (100 mL water, 0.6 mL HCl, 1 g K2S2O5) was used to color the ferritic matrix of annealed T15 and M42 and to color the martensitic matrix in the M42 specimens. Vilella’s reagent was used to darken the martensite in the D3 specimen. Beraha’s Sulfamic acid reagent (100 mL water, 3 g K2S2O5, 2 g Sulfamic acid and 1 g NH4·FHF) was used to color the ferritic annealed matrix of the T1 and M42 specimens. Other reagents were tried, but these yielded the best contrast between the unaffected carbides and the colored matrix while yielding crisp grain boundaries. The specimens were evaluated by automated image analysis to determine the volume fraction, number per unit area and size of the carbides. Next, the specimens were re-prepared and etched with the reagents listed in Table 3. Results of the etching experiments are shown in Table 4.
 Most of the selective carbide etchants produced higher volume fractions and greater numbers of particles than were obtained when the matrix was darkened by etching. In a few cases, the results were close, as was the case for the M7C3 carbides in the D3 specimen etched with Murakami’s and Groesbeck’s reagents compared to the measurement made when the matrix was darkened. But, this was the exception rather than the rule.
Most of the selective carbide etchants produced higher volume fractions and greater numbers of particles than were obtained when the matrix was darkened by etching. In a few cases, the results were close, as was the case for the M7C3 carbides in the D3 specimen etched with Murakami’s and Groesbeck’s reagents compared to the measurement made when the matrix was darkened. But, this was the exception rather than the rule.
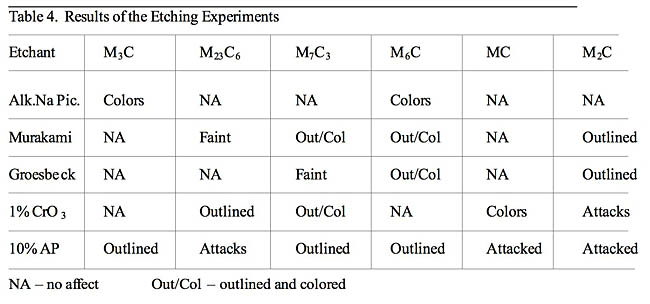 The reagents do have reasonable selectivity and can be used to identify carbides when sophisticated electron instruments are not available. The results frequently agreed with the literature, but differences were observed. Alkaline sodium picrate colored the Fe3C and M6C carbides, and nothing else, as claimed. Murakami’s at room temperature outlined and colored Cr7C3 carbides in D3 rather than attacking them and attacked the Cr23C6 carbides in 440C instead of faintly revealing them. Groesbeck’s reagent did not attack the Cr23C6 carbides and did not reveal them. It faintly revealed the Cr7C3 carbides and it outlined the M2C carbides rather than attacking them. Electrolytic CrO3 outlined Cr7C3 carbide, as stated, but did not attack them, as claimed. It did not outline M2C carbides but attacked them. Electrolytic 10% ammonium persulfate has not been studied in the past as to its selectivity. It has mainly been used to color Cr23C6 carbide in sensitized austenitic stainless steels. In this study, it outlined Fe3C, M6C and Cr7C3 carbides and attacked Cr23C6, M2C and MC carbides. In general, they are not satisfactory for quantitative metallographic measurements as bias was obtained in the volume fractions, number of particles per unit area and their size.
The reagents do have reasonable selectivity and can be used to identify carbides when sophisticated electron instruments are not available. The results frequently agreed with the literature, but differences were observed. Alkaline sodium picrate colored the Fe3C and M6C carbides, and nothing else, as claimed. Murakami’s at room temperature outlined and colored Cr7C3 carbides in D3 rather than attacking them and attacked the Cr23C6 carbides in 440C instead of faintly revealing them. Groesbeck’s reagent did not attack the Cr23C6 carbides and did not reveal them. It faintly revealed the Cr7C3 carbides and it outlined the M2C carbides rather than attacking them. Electrolytic CrO3 outlined Cr7C3 carbide, as stated, but did not attack them, as claimed. It did not outline M2C carbides but attacked them. Electrolytic 10% ammonium persulfate has not been studied in the past as to its selectivity. It has mainly been used to color Cr23C6 carbide in sensitized austenitic stainless steels. In this study, it outlined Fe3C, M6C and Cr7C3 carbides and attacked Cr23C6, M2C and MC carbides. In general, they are not satisfactory for quantitative metallographic measurements as bias was obtained in the volume fractions, number of particles per unit area and their size.
George Vander Voorthas a background in physical, process and mechanical metallurgy and has been performing metallographic studies for 43 years. He is a long-time member of ASTM Committee E-4 on metallography and has published extensively in metallography and failure analysis. He regularly teaches MEI courses for ASM International and is now doing webinars. He is a consultant for Struers Inc. and will be teaching courses soon for them. He can be reached at 1-847-623-7648, EMAIL: georgevandervoort (AT) yahoo (DOT) com and through his web site: www.georgevandervoort.com
To View a listing of all George’s articles please click here
Read George Vander Voort’s Biography
