Introduction
Nitriding is one of the most interesting and useful surface-hardening techniques. It is unique in that during the nitriding process, the specimen is not heated into the austenite phase, and it does not rely upon the formation of martensite to achieve high hardness and useful properties. It is heat treated prior to nitriding, forming tempered martensite to obtain the desired core properties unlike all other surface heat-treatment processes.
The processing associated with nitriding does have some advantages in avoiding problems such as quench cracking and distortion. It also has some side benefits in improved corrosion resistance and generation of beneficial residual compressive stresses, which improves fatigue resistance. Nitrided surfaces do exhibit high surface hardness, leading to improved wear resistance.
Of course, like any process, there are disadvantages. One that has always plagued nitriding is the very long cycle time to achieve a decent case depth, about 20 times longer than for carburizing considering equal case depths. The other problem has been the generation of the compound layer, often erroneously called the “white-etching layer,” which is brittle and is generally deleterious if present. Etching does not color this layer white; it is white, just as it was as-polished, since the etchant had no effect on the layer.
Literature Review
 |
|
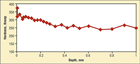
Fig. 2. Knoop hardness profile (100 gf load) starting as close as possible to the compound layer to a depth of 1 mm
|
The literature on the microstructure of nitrided steels does, unfortunately, contain numerous errors and could be improved. Examples of the microstructures of nitrided steels in the literature are often subpar and even poor or false. Phase identification of the compound layer and the underlying diffusion zone need work.
In the past, bulk X-ray diffraction on the OD surface has been the chief tool for phase identification. While a useful tool, it needs augmentation by microanalytical methods with very small spatial resolution for phase identification. Limited work has been done using well-established tools such as the transmission electron microscope. This is partly due to the difficulty in preparing thin foils from relatively small regions at the surface compared to the ease in preparing foils from bulk specimens. Also, it is somewhat a reflection of the types of research studies being funded at universities in the U.S. The writer is currently exploring the use of electron backscattered diffraction (EBSD) with the scanning electron microscope (SEM) to see if this technique can provide an easier approach for analysis of the compound layer.
In the literature, one can see nitrided examples of low-carbon steels; low-carbon, low-alloy steels; and HSLA (high-strength, low-alloy) steels (none of these contain significant amounts of alloying elements that form hard nitrides, such as Al, Cr, V or Mo). They exhibit an outer layer, called the “compound layer,” which is reported to be composed of iron nitrides: ?’ (Fe4N) and e (Fe2N1-x) phases.
Little, if any, solid-solution strengthening occurs from the diffused nitrogen into the steel. Some needle-like intragranular iron nitrides, probably ?’, may be seen in ferrite grains below the compound layer, but they have little influence on the case hardness. Consequently, nitriding has been mainly centered upon steels that contain elements that form very fine, very hard nitrides: Al, Cr, V and Mo. Elements such as Ti and Zr do form very hard nitrides in steels, but they are comparatively quite large (in the µm range) and do not create a case-hardness profile.
The original experiments[1] on nitriding performed by Adolph Machlet at the American Gas Company in Elizabeth, N.J., (see U.S. Patent 1,092,925, dated 24 June 1913) focused on nitriding carbon steels. In 1906, Adolph Fry of the Krupp Steel Works in Essen, Germany, began a similar study of nitriding.[1] However, Fry realized early in his work that alloying elements were necessary to develop commercially useful nitrided steels. Fry’s U.S. patent (1,487,554) was granted on March 18, 1924. Fry learned that only steels containing additions of Cr, Mo, Al, V or W could achieve a high surface hardness when nitrided. His work at Krupp led to the development of the Nitralloy grades.
 |
|
Fig. 3. Composite image made from several contiguous fields to show the case/core microstructure of the Nitralloy 135 specimen, etched with a 10:1 mix of 4% picral and 2% nital (originals at 200X)
|
Etchants
A perusal of publications regarding nitriding reveals a range of etchants that have been used. Nital, by far the most widely used etchant for steels, has been commonly used for nitrided steels. McQuaid and Ketcham used 4% nital to etch nitrided Cr-Al and Mo-Al steels and AISI/SAE 4615 in their study published in 1928.[1] Robert Sergeson, a research metallurgist at the Central Alloy Steel Corporation in Canton, Ohio, and an early researcher of the nitriding process, introduced the use of a 10-to-1 solution of 4% picral plus 4% nital in 1929 (although many people have used 2% nital instead of 4%).[2] This is an excellent etchant.
Lightfoot and Jack[3] studied nitriding with and without formation of the compound layer. They noted that during nitriding a carbon-rich layer is created ahead of the nitrided case. This carbon precipitates as cementite in grain boundaries that are roughly parallel to the surface. This carbon accumulation caused by the inward diffusion of nitrogen has been verified by others.[4-6] Jegou et al.[6] used the electron microprobe to measure the nitrogen and carbon profiles through the nitrided case. These authors showed the grain-boundary films darken in specimens nitrided for 10 and 100 hours when they etched with ”boiling picral.” They probably used boiling alkaline sodium picrate.
It is well known that alkaline sodium picrate used at 80-100°C will darken cementite (Fe3C) carbide. Albert Sauveur, dean of American metallurgists, attributed[7] the boiling alkaline sodium-picrate etch for identification of cementite to Kourbatoff in 1906. Despite studies proving that the white grain-boundary films in the diffusion zone are cementite, numerous authors have stated that they are nitride (Reference 8, for example). This seems to be a common natural error, assuming that the white grain-boundary film is a nitride just like the compound layer.
The only systematic study on the use of etchants to identify the phases in the compound zone and the white grain-boundary films is by Mridha and Jack.[9] They evaluated 10 different etchants and showed that for nitrided pure iron, nital does not distinguish the phases in the compound zone. They concluded that the best reagents to distinguish ?’ from e are picral (etches boundaries in the nitride phases), Vilella’s reagent (attacks boundaries in e and stains ?’), a sulfate-chloride solution (stains only e) and Oberhoffer’s reagent (a short 2-5 second etch dissolves e).
For alloy steels (chiefly 3% Cr steels), the boundary of the nitrided zone was best revealed using picral, Marble’s and Oberhoffer’s reagents. The latter two etchants best revealed the extent of the carbide-enriched region beneath the nitrided region. The sulfate-chloride reagent was sensitive to all constituents. The best etchant for revealing the white grain-boundary films of cementite was alkaline sodium picrate used at 85°C for 2 minutes. This etch also revealed the presence of cementite in the compound zone. Cementite was confirmed using X-ray diffraction. They recommended etching nitrided steels first with alkaline sodium picrate and then with Oberhoffer’s reagent for 3 seconds. Reference 10 by the same authors covers the characterization of nitrided 3% Cr steels using etchants selected based upon this study.[9]
Microstructures
An example of a low-carbon, resulfurized steel – AISI/SAE 1215 – that was salt-bath nitrided is shown in Figure 1. This specimen was etched with a 10-to-1 mix of 4% picral to 2% nital, an etchant that has often been used to reveal the structure of nitrided steels. Note that we see a well-developed compound layer. Because 1215 has no appreciable content of alloying elements that would form alloy nitrides (Al, Cr, V, Mo or W), there is no diffusion zone (as shown later for Nitralloy 135 and 41B50). Note the iron-nitride “needles” (arrows) intragranular within the ferrite grains beneath the compound layer.
Figure 2 shows a plot of Knoop hardness (100 gf load) versus depth curve starting as close as possible to the compound layer. This layer is too thin to actually test, so the first indent is not able to evaluate the actual hardness of the compound layer. But one can see from the rest of the data that nitriding has had only a minor influence on the case hardness.
 |
|
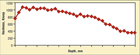
Fig. 6. Knoop hardness profile plot (100 gf load) for Nitralloy 135, free of any compound zone, showing a very high hardness case
|
Figure 3 shows the case/core microstructure of gas-nitrided Nitralloy 135, an alloy developed by Fry’s work reported above, which contains ~1.1% Al, ~1.6% Cr and ~0.2% Mo – good nitride formers. Note that this specimen does not exhibit a compound layer. With today’s digital technology, we can create a mosaic image from a series of images of contiguous fields, where we control the lighting and then “weld” the digital images together with appropriate software. This is marvelous technology compared to the older, very painful practice of trying to glue prints taken in a similar alignment, which can no longer be done.
Figure 4 shows the white grain-boundary films in the diffusion zone that have often been erroneously identified as nitrides (in studies without any analytical work) because they are white. Figure 5 shows that these grain boundaries are darkened when etched with hot alkaline sodium picrate, proving that they are cementite. Figure 6 shows the Knoop hardness (100 gf load) profile for this specimen – markedly better than for the 1215 carbon steel shown in Figure 2.
A more complex example of a nitrided alloy steel, resulfurized 41B50, is shown in Figure 7. This was a chuck jaw made for a lathe that broke as soon as it was put into service due to the brittle nature of the surface layer. The specimen was electroless nickel-plated to enhance edge retention.
Figure 7 shows the surface in the as-polished condition. Note the MnS stringers in this lightly resulfurized alloy steel. Three zones can be seen in the complex compound layer. This type of complex compound zone has been reported in the literature, but it is not common. Zone 1 is believed to be epsilon phase formed by outward diffusion of Fe along pore channels and reaction with N at the surface. Zone 2 is believed to be porosity in the epsilon phase (according to the literature), which may be filled with oxide.
 |
|
Fig. 8. Failed 41B50 chuck jaw with a nitrided surface exhibiting a massive compound layer etched with 10:1 mix of 4% picral and 2% nital (original at 500X)
|
Examination of this zone with dark-field illumination did not confirm that the black spots are holes, however, nor did the inital SEM examination at high magnification. So, more work is needed to positively identify this dark portion of the compound zone.
Zone 3 is the classic mixture of epsilon and gamma-prime phase. In the microscope there is a slight dark/light contrast difference between the two phases, which can be faintly seen in the micrograph. Figure 8 shows the complex compound zone after etching with the 10:1 mixture of 4% picral to 2% nital. Note that detail is revealed in the compound zone (Zone 3).
Figure 9 shows the compound zone and diffusion zone after etching with 10% sodium metabisulfite. There is what appears to be oxidation between the electroless nickel plating and zone 1, as also shown in Figures 7 and 8. Figure 10 shows two views of the complex compound layer and the diffusion zone of the failed nitrided part after etching with alkaline sodium picrate at 90°C for 90 seconds to color the cementite. Note that the lower edge of the compound zone (area 3) contains cementite, as reported by previous researchers. The white grain-boundary films are darkened by the alkaline sodium-picrate etch while the coarser cementite in zone 3 of the compound layer is a mix of blue and blackish particles.
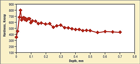
Figure 11 shows the Knoop hardness (100 gf load) profile for the failed nitrided 41B50 chuck-jaw specimen. Note that the hardness in the outer dark surface (zone 2) of the compound zone is lower than the inner (zone 3) layer. Zone 1 is far too thin to determine its hardness accurately. 41B50 contains ~0.95% Cr and ~0.20% Mo, but neglible Al (a small amount may be present for grain refinement). Note that the maximum case hardness obtained in the nitrided 41B50 specimen is much lower (~620-690 HK) than obtained for the nitrided Nitralloy 135 specimen (~1000-1040 HK) with ~1.1% Al, 1.6% Cr and ~0.2% Mo (Fig. 11 compared to Fig. 6). Both cases, however, are markedly harder than that of the nitrided 1215 carbon steel (~300-340 HK) in Fig. 2. This shows just how critical it is to develop an alloy composition that will form very hard, very small nitrides.
Conclusions
Metallography, when properly performed, is an exceptionally important tool for studying the microstructure of nitrided steels, as well as other heat-treated metals and alloys. All etchants are not equal, and nital is not always the best etch for all steels, despite its wide usage. Different steel compositions do respond quite differently to nitriding, as illustrated by the comparison of a nitrided carbon steel with two alloy steels – one with a greater concentration of alloying elements that will form very fine, hard nitrides compared to the leaner alloy. The Knoop hardness profiles for these three steels were markedly different.
 |
|
Fig. 11. Knoop hardness (100 gf load) profile of the failed nitrided 41B50 chuck-jaw specimen revealing a low hardness in the complex compound layer
|
Nitriding processes must be controlled to eliminate the brittle compound layer, which has been known to cause failures when present. The work shows that the compound layer can be rather variable in appearance. It is also common to see white grain-boundary films only in the boundaries that are parallel or nearly parallel to the specimen surface. These films have frequently been claimed to be nitrides, but numerous studies have proven that they are cementite.
The exact mechanism for the formation of these films has not been fully defined, although there are a few good preliminary studies. It appears that as nitrogen is diffused into the steel, carbon is pushed from the surface inward. Only limited electron microprobe (EMPA) work has been done to study the C and N case profiles, but these show that the C is depleted at the surface and pushed inward while the N content is highest at the surface and drops as the case hardness decreases. Application of good analytical techniques, such as EBSD and the EMPA, in future studies should enhance our understanding of the nitriding process.
References
2. R. Sergeson, “Investigations in Nitriding,” ASST Nitriding Symposium, 1929 (republished in the Source Book on Nitriding, American Society for Metals, Metals Park, Ohio, 1977, pp. 26-55).
3. B.J. Lightfoot and D.H. Jack, “Kinetics of Nitriding With and Without White-Layer Formation,” Heat Treatment ’73, The Metals Society, December 1973 (republished in the Source Book on Nitriding, American Society for Metals, Metals Park, Ohio, 1977, pp.248-254).
4. L. Barrallier et al., “Morphology of Intergranular Cementite Arrays in Nitrided Chromium-Alloyed Steels,” Materials Science and Engineering, Vol. A393, 2005, pp. 247-253.
5. V. Yu. Traskine et al., “Physicochemical Mechanics of Structural Transformations in Nitrided Steel,” Colloid Journal, Vol. 67, No. 1, 2005, pp. 97-102.
6. S. Jegou, L. Barrallier, R. Kubler and M.A.J. Somers, “Evolution of Residual Stress in the Diffusion Zone of a Model Fe-Cr-C Alloy During Nitriding,” HTM J. Heat Treatment Mat., Vol. 66, No. 3, 2011, pp. 1-8.
7. A. Sauveur, The Metallography and Heat Treatment of Iron and Steel, 4th ed., McGraw-Hill Book Co., NY, 1935, pgs. 482, 502 and 504.
8. R. Agnelli et al., “Failure Analysis in Tool Steels,” Failure Analysis of Heat Treated Steel Components, L.C.F Canale, R.A. Mesquita and G.E. Totten editors, ASM International, Materials Park, Ohio, 2008, pp. 311-350.
9. S. Mridha and D.H. Jack, “Etching Techniques for Nitrided Irons and Steels,” Metallography, Vol. 15, No. 2, May 1982, pp. 163-175.
10. S. Mridha and D.H. Jack, “Characterization of Nitrided 3% Chromium Steel,” Metal Science, Vol. 16, August 1982, pp. 398-404.
George Vander Voorthas a background in physical, process and mechanical metallurgy and has been performing metallographic studies for 43 years. He is a long-time member of ASTM Committee E-4 on metallography and has published extensively in metallography and failure analysis. He regularly teaches MEI courses for ASM International and is now doing webinars. He is a consultant for Struers Inc. and will be teaching courses soon for them. He can be reached at 1-847-623-7648, EMAIL: georgevandervoort (AT) yahoo (DOT) com and through his web site: www.georgevandervoort.com
To View a listing of all George’s articles please click here
Read George Vander Voort’s Biography





