ABSTRACT
Experiments were conducted using three-step preparation procedures for titanium and its alloys. For CP titanium and alpha-titanium alloys, use of an attack-polishing agent in the third step was required to obtain good results. The experiments defined optimum surfaces for each step and operating conditions. Two-phase, α-ß alloy specimens are significantly easier to prepare than a single-phase α specimen. The method does yield perfect polarized light response with α-phase alloys, such as commercial-purity titanium.
INTRODUCTION
Titanium and its alloys have become quite important commercially over the past fifty years due to their low density, good strength-to-weight ratio, excellent corrosion resistance and good mechanical properties. On the negative side, the alloys are expensive to produce. Titanium, like iron, is allotropic and this produces many heat treatment similarities with steels. Moreover, the influences of alloying elements are assessed in like manner regarding their ability to stabilize the low temperature phase, alpha, or the high temperature phase, beta. Like steels, Ti and its alloys are generally characterized by their stable room temperature phases – alpha alloys, alpha-beta alloys and beta alloys, but with two additional categories: near alpha and near beta.
Titanium and its alloys are more difficult to prepare for metallographic examination than steels. They have low grinding and polishing rates. Deformation twinning can be induced in alpha alloys by over aggressive sectioning and grinding procedures. It is safest to mount relatively pure Ti specimens, especially those from service in a hydrogen-containing environment, in castable (“cold”) resins rather than using hot compression mounting due to the potential for altering the hydride content and morphology. If hydride formation is unlikely, then a hot-mounting compound may be used. Elimination of smearing and scratches can be quite difficult.
Early mechanical preparation procedures [1-5] tended to be rather long, involved procedures nearly always incorporating an attack polishing solution in the last step or last two steps. Some of the more commonly used attack polishing solutions are summarized in [6]. The problems associated with obtaining well-prepared surfaces have prompted considerable interest in electropolishing procedures [3-5, 7, 8]. The inherent danger of some of these electrolytes has prompted interest in chemical polishing procedures [9]. Electrolytic and chemical polishing solutions for Ti and Ti alloys are also summarized in [6].
Mechanical polishing methods for titanium and its alloys continued to rely upon these older procedures into the 1970’s [10] and 1980’s [11]. Perhaps the first publication of a modern approach to preparing titanium was that of Springer and Ahmed [12] in 1984. This was a three-step procedure, assuming that the planar grinding step can be performed with 320-grit SiC paper, which may not always be possible. If the specimens are sectioned using a wafering blade or an abrasive blade of the proper bond strength, which produce a smooth surface with minimal damage, then 320-grit SiC paper may be used. If a rougher surface with greater damage is produced, such as would result from use of a power hacksaw, then grinding must commence with a coarser grit paper in order to remove the damage in a reasonable time. In general, if a specimen was sectioned with a power hacksaw or a band saw, it is best to re-section it using an abrasive saw with a blade developed for Ti, or a precision saw as the damage produced by a band saw or power hack saw will be extensive and difficult to remove by grinding. The Secotom saw is ideal for Ti and its alloys.
The procedure developed by Springer and Ahmed was:
- Wet grind with 320-grit SiC paper for 2-3 minutes to obtain a flat surface free of cutting damage.
- Rough polish with 9-µm diamond paste on a perforated synthetic chemotextile pad for 10-15 minutes with distilled water as lubricant.
- Final polish with 0.02-µm colloidal silica suspension on a medium nap cloth for 10-15 minutes.
G. Müller, as reported by Leistner [13], also developed a three-step procedure for titanium. Again, it will take three steps only if sectioning produces a smooth surface with minimal damage. His procedure was:
- Wet grind with P500 SiC paper, 300 rpm, 100 N load (6 specimens), until all surfaces are co-planar.
- Wet grind with P1200 SiC paper, 300 rpm, 100 N load, for 30 seconds.
- Polish with colloidal silica suspension containing an attach polishing agent on a synthetic napless cloth, 150 rpm, for: 10 min. at 200 N, 2 min. at 100 N, 2 min. at 50 N, and 1 min. without any load.
The polishing suspension consisted of 260 mL of colloidal silica, 40 mL H2O2 (30%), 1 mL HNO3 and 0.5 mL HF.
PREPARATION EXPERIMENTS
Method of Springer and Ahmed
The initial preparation experiments centered on evaluating the method of Springer and Ahmed [12] (S&A). As the perforated cloth that they used was no longer available, other surfaces had to be evaluated. Also, the published method did not specify the applied load, platen rpm or rotational directions.
The first experiment with the S&A method used:
- Grind with 320-grit SiC paper, water cooled, 240 rpm, complementary* rotation, 27 N (6 lbs.) per specimen load, until plane.
- Rough polish with 9-µm diamond on a flat, hard-woven nylon cloth, 120 rpm, 27 N/specimen, with a lubricant added, contra** rotation, 10 min.
- Final polish with 0.02-µm colloidal silica on a medium nap polishing cloth, 120 rpm, contra rotation, 10 minutes.
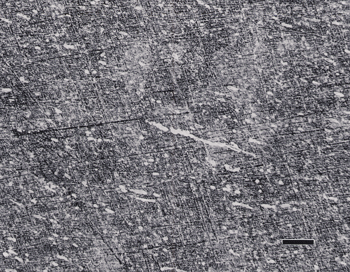
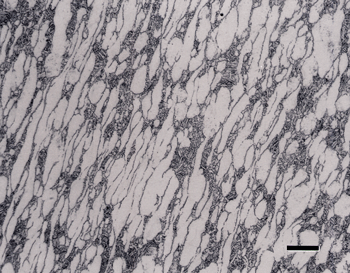
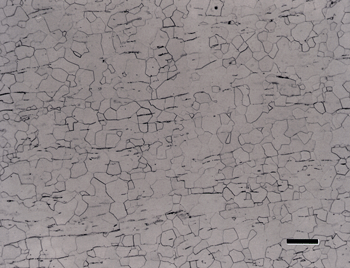
If the head speed is greater than about 100 rpm, slurries will be thrown off the platen surface, and contra rotation should not be used. Use 60 rpm for the head in contra rotation mode. Contra is about 10% more aggressive in removal rate than complementary and the abrasive and lubricant stay on the cloth longer. Results for titanium alloys were generally acceptable, although some scratches were observed; but they were inadequate for CP titanium, as shown in Figure 1.
Addition of a 3-µm diamond step between steps 2 and 3 did not improve the alpha alloy and the CP Ti microstructures. Whether the cloth used was hard and napless, or softer and napped, the results were the same for the CP Ti specimens. The use of a woven silk cloth in step 2 resulted in fewer scratches after this step and yielded acceptable results for the CP Ti specimens. A synthetic chemotextile pad was also tried for step 2 and found to produce nearly as good results as with the silk cloth.
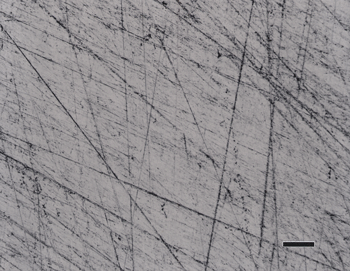
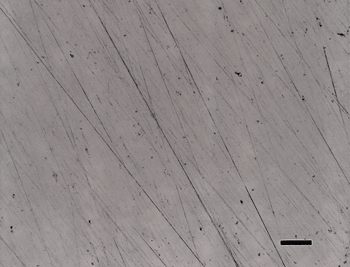
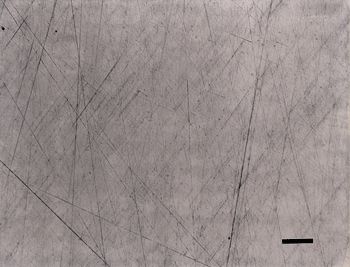
Figure 2 shows the surface of the same specimen after grinding with 320-grit SiC (step 1) and then rough polishing (step 2) with 9-µm diamond on a) a coarse, heavy cloth similar to sailcloth, b) the flat, woven silk cloth; and, c) the synthetic chemotextile pad. Based on these experiments, the flat, woven silk cloth was selected as the preferred cloth for step 2 and the synthetic chemotextile pad was considered to be nearly equivalent.
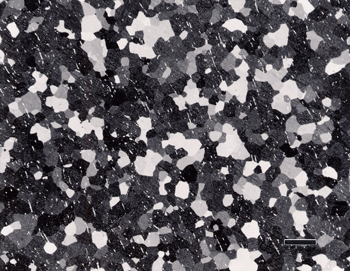
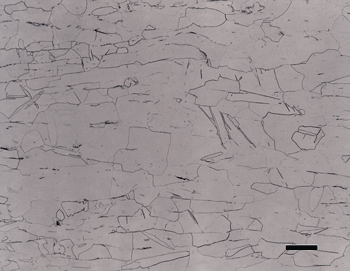
As an example of these experiments, Figure 3 shows the microstructure of two specimens using flat, woven silk cloth for step 2 and a medium nap, flocked cloth for step 3. Etching with aqueous 0.5% HF produced colored alpha grains on CP Ti (Figure 3b), which is not normal. Higher magnification examination revealed some roughness in the alpha grains. Using a different medium nap, flocked cloth for step 3 produced excellent results. If Kroll’s reagent is used, instead of 0.5% HF, a flat grain boundary etch for the CP Ti specimens is obtained rather than a color grain contrast etch, but only when the preparation is near perfect. Figure 4 shows an alpha alloy and a CP Ti specimen. The two-phase alloys were well prepared but the alpha alloy and the CP Ti specimens were inadequate.
2.2. Addition of an Attack-Polishing Agent
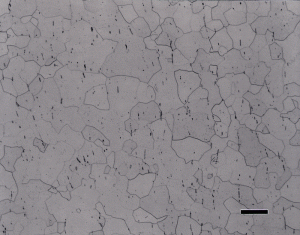
If an attack polishing solution is added to the colloidal silica abrasive for step 3, the structures are all revealed more clearly, as Figure 5 shows. In this experiment, step 2 used the flat, woven silk cloth and step 3 used the second medium nap, flocked cloth while the abrasive consisted of: 50mL 0.02 μm colloidal silica, 10mL H2O2 (30%) and 5mL Kroll’s reagent. Figure 5b shows a well developed alpha grain structure in an annealed CP Ti specimen with little cold work in the alpha grains.
This experiment, and numerous subsequent experiments, revealed that an attack-polishing agent must be used in step 3 to get satisfactory images of alpha alloys and CP Ti specimens. For step 3, napped cloths yield better results for CP Ti than harder, napless polyurethane cloths. For edge retention and cavity wall retention in cast alloys, the polyurethane cloths will produce better results and they hold up better when using an attack polishing agent in the abrasive. All of these cloths work well for two-phase Ti alloys, such as the popular Ti-6Al-4V.
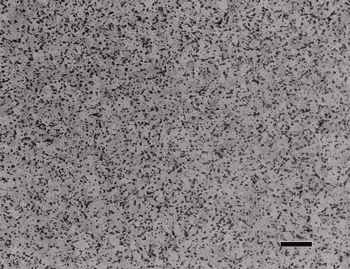
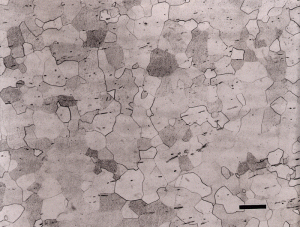
If a synthetic chemotextile pad is used for step 2, a medium-nap flocked polishing cloth is used for step 3, and attack polishing is employed, results are still excellent, as shown in Figure 6.
2.3. Method of Müller
The three-step procedure of G. Müller (13) was also tried. The results were not as good as, there was a bit more cold work visible in the alpha grains of the CP Ti specimens. Figure 7 shows an example of one of the best CP Ti structures with this procedure.
2.3. Other Variations
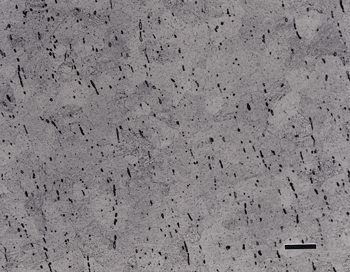
Two final three-step preparation procedures were tried. First, 240-grit SiC paper was used for step 1 rather than 320-grit paper. Most of the alpha-beta alloy specimens were prepared adequately, although some exhibited excessive cold work in the alpha phase, as in Ti-6Al-4V containing equiaxed alpha. The other experiment involved substitutions of 0.05-µm gamma alumina for colloidal silica in step 3 (using the same attack polishing additive). Results were generally acceptable, especially for the two-phase alloys, but the alpha phase in CP Ti specimens contained substantial cold work, Figure 8.
A few variants of the attack polishing solution were tried. Leonhardt [14] uses a mixture of: 150mL colloidal silica, 150mL water, 30mL H2O2 (30%), 1-5 mL HF and 1-5 mL HNO3. Results with this attack polishing additive to the abrasive were equivalent to the one used. Buchheit [6] added 5 mL of a 20% aqueous CrO3 solution to 30 mL of an alumina slurry. To try this, but using colloidal silica instead, 10 mL of the 20% CrO3 solution was added to 75 mL of colloidal silica. This also produced excellent results. In using these attack polishing solutions, care must be taken in handling, mixing and using these additives as they contain very strong oxidizers and acids. Avoid physical contact with the ingredients, especially HF which produces serious injury if it contacts skin, and the prepared attack polishing abrasives.
3. CONCLUSIONS
A three-step procedure can be used quite successfully to prepare titanium and titanium alloys. Use of an attack polish additive in step 3 is required to obtain good results, particularly with CP titanium and alpha Ti alloys. Most two-phase Ti alloys can be satisfactorily prepared without using an attack-polishing additive, although results were better when it was used. Optimal and alternative surfaces and operating conditions were defined.
Table 1. Three-Step Preparation Procedure for Titanium and Its Alloys.
- Wet grind with 320-grit SiC paper, 30N load per specimen, complementary rotation, 240 rpm, 1 minute.
- Rough Polish with 9-µm diamond paste on a flat, woven silk cloth, such as MD-Dac, 30N load per specimen, 120 rpm for the platen, contra rotation (head at 60 rpm), adding Red or Green lubricant periodically (or use a 9-µm diamond suspension), for 10 minutes.
- Final polish with a mix of 5 parts OP-S colloidal silica and 1 part H2O2 (30% conc.) on a medium-nap polishing cloth, such as an MD-Floc cloth, 30N per specimen, 120 rpm platen speed, contra rotation (60 rpm head speed), for 10 minutes.
Note: For high-purity Ti (99.99% Ti), add a 3-μm step after the 9-µm step, in the same manner, but for 5 minutes.
REFERENCES
- Finlay, W. L., Resketo, J. and Vordahl, M. B., “Optical Metallography of Titanium”, Industrial and Engineering Chemistry, Vol. 42, p.218-222 (February 1950).
- Craver, C. B., “Differentiation of Grain Size and Phases in Titanium”, Metal Progress, Vol. 59, p. 371-373 (March 1951).
- Osadchuk, R., Koster, W. P. and Kahles, J. F., “Recommended Techniques for Polishing Titanium for Metallographic Examination,” Metal Progress, Vol. 64, p.129-131, 236, 240 (October 1953).
- McQuillan, A. D. and McQuillan, M. K., Titanium, Academic Press, N.Y., p.447-458 (1959).
- Echer, C. J., Cooney, J. E. and Kish, A. J., “Metallography of 5Al-2.5Sn Titanium Alloy”, Metals Engineering Quarterly, Vol. 7, p.58-63 (February 1967).
- Vander Voort, G. F., Metallography: Principles and Practice, McGraw-Hill, N.Y., p.548 (1984); ASM International, Materials Park, Ohio (1999).
- Jacquet, P. A., “Electrolytic Polishing and Oxidation of Titanium”, Metal Treatment and Drop Forging, Vol. 18, p. 176, 182 (April 1951).
- Coons, W. C. and Isoty, L. R., “Electrolytic Polishing System for Space Age Materials”, Metal Progress, Vol. 109, p.36-40 (May 1976).
- Simmer, B. and Schmalfuss, D., “Comments on The Preparation of Metallographic Specimens of Titanium with Particular Reference to Chemical Polishing”, Praktische Metallographie, Vol. 15, p.78-85 (February 1978).
- “Metallographic Techniques for Titanium and Titanium Alloys”, Metals Handbook, 8th ed., Metallography, Structures and Phase Diagrams, ASM, Metals Park, OH, Vol. 8, p.140-141 (1973).
- Boyer, R. R., “Titanium and Titanium Alloys”, Metals Handbook, 9th ed., Metallography and Microstructures, Vol. 9, ASM, Metals Park, OH, p.458-475 (1985).
- Springer, C. and Ahmed, W. U., “Metallographic Preparation of Titanium”, Praktische Metallographie, vol. 21, p.200-203 (1984).
- Leistner, E., “Automatic Specimen Preparation for Titanium – Couldn’t be Better”, Structure, No. 17, p.12-13 (June 1988).
- Leonhardt, T., Rhenium Alloys, private communication.
George Vander Voort has a background in physical, process and mechanical metallurgy and has been performing metallographic studies for 47 years. He is a long-time member of ASTM Committee E-4 on metallography and has published extensively in metallography and failure analysis. He regularly teaches MEI courses for ASM International and is now doing webinars. He is a consultant for Struers Inc. and will be teaching courses soon for them. He can be reached at 1-847-623-7648, EMAIL: georgevandervoort@yahoo.com and through his web site: www.georgevandervoort.com
To View a listing of all George’s articles please click here
Read George Vander Voort’s Biography