The metallographic laboratory is a relatively safe working environment; however, there are dangers inherent to the job. Included in these dangers is exposure to heat, acids, bases, oxidizers and solvents. Specimen preparation devices, such as drill presses, shears and cutoff saws, also present hazards. In general, these dangers can be minimized if the metallographer consults documents such as ASTM E 2014 (Standard Guide on Metallographic Laboratory Safety) and relevant Material Safety Data Sheets (MSDS) before working with unfamiliar chemicals. Common sense, caution, training in basic laboratory skills, a laboratory safety program, access to safety reference books – these are some of the ingredients of a recipe for laboratory safety. Table 1 lists the main requirements for a comprehensive safety program.
 |
| Table 1. Elements of a Comprehensive Metallography Laboratory Safety Plan. |
Safe working habits begin with good housekeeping. A neat, orderly laboratory promotes safe working habits, while a sloppy, messy work area invites disaster. Good working habits include such obvious, commonsense items as washing the hands after handling chemicals or before eating. Simple carelessness can cause accidents (Fig.1). For example, failure to clean glassware after use can cause an accident for the next user. Another common problem is burns due to failure to properly clean acid spills or splatter.
Most reagents, chemical or electrolytic polishing electrolytes, and solvents should be used under a ventilation hood designed for use with chemicals. Many of these chemicals used in metallography can cause serious damage on contact. It is best to assume that all chemicals are toxic and all vapors or fumes will be toxic if inhaled or will be damaging to the eyes. A hood will prevent the working area from being contaminated with these fumes. However, you will not be protected when your head is inside the hood. In most cases, a protective plastic or shatterproof glass shield can be drawn across the front of the hood for further protection from splattering or any unexpected reactions.
All laboratories should be equipped with a shower and eyewash, Fig. 2, for emergency use. This device should be near the work area so that the injured can reach it quickly and easily. Fire alarms and fire extinguishers (CO2 type) should be available and tested periodically. A good first aid kit and a chemical spill treatment kit should be readily available. People must be trained in how to use the first aid kit and chemical spill treatment kit. There is no time to read the instructions when an accident occurs.
Metallography Laboratory Equipment
Specimen preparation devices used in metallographic laboratories are generally quite safe to use, as most are designed and constructed to include safety features. Information supplied by the manufacturer usually describes safe operating procedures. It is good laboratory practice to prepare a job safety analysis detailing potential hazards and describing the safe operating procedure for each piece of equipment. This information should be provided to all users and it must be revised and reviewed periodically.
Band saws or abrasive cutoff saws are commonly used by metallographers. The cutting area of band saws is exposed and potentially dangerous. Particles can fly off, so safety glasses are a must. Your hands should never be used to guide the work piece during cutting. A guiding device or block of wood should always be used between the work piece and your hands. After cutting is completed, the saw should be turned off before pieces near the blade are removed. Wear proper gloves designed for cutting (not disposable plastic gloves). Samples should be handled carefully, because considerable heat can be generated. In addition, sharp burrs are often present, which should be carefully removed by filing or grinding. Abrasive cutoff saws are safer to use because the cutting area is closed off during use. The chief danger is from flying pieces from a broken wheel. Fortunately, the closed cover contains these pieces within the cutting chamber. Wheel breakage usually occurs when the part is not firmly clamped in place or if excessive pressure is applied, a bad practice from the standpoint of specimen damage as well.
Dust produced during grinding of metals is always dangerous. For certain metals, like beryllium, magnesium, lead, manganese, and silver, the dusts are extremely toxic. Wet grinding is preferred both for dust control and for preventing thermal damage to the specimen. Bench grinders must be firmly mounted to prevent sudden movement. Care must be exercised to avoid grinding one’s fingers or striking the edge of a grinding belt, which will cause painful lacerations. With nearly all materials, wet grinding is preferred and produces best results. For routine handling of dangerous metals, grinding should be done wet under a ventilation hood. Waste must be handled carefully and disposed of properly. Radioactive materials require special remote-handling facilities and elaborate safety precautions.
A drill press is sometimes used in the laboratory. Drilling holes in thin sections requires secure clamping; otherwise the sample can be grabbed by the drill and spun around, inflicting serious lacerations. Hair, ties, and shirt cuffs can become entangled in a drill, inflicting serious injuries. Safety glasses should always be worn when using drill presses or when cutting or grinding. Mounting presses or laboratory heat-treatment furnaces present potential burn hazards. Gloves of the correct type should be worn when working with these devices. Modern mounting presses that cool the cured resin back to near room temperature dramatically reduce the potential for burns. It is a good practice to place a “hot” sign in front of a laboratory furnace when it is in use.
 |
| Figure 1. Carelessness in the laboratory can result in dangerous chemical spills and a clean-up nightmare. |
It is occasionally necessary to heat solutions during their preparation or use. Although Bunsen burners have been used in the past for this purpose, it is much safer to use a hot plate or water bath and thus avoid the use of an open flame. If a Bunsen burner is used, the flame should never be applied directly to a flask, beaker, or dish. Plain- or asbestos-centered wire gauze should always be placed between the flame and the container.
Personal Protective Equipment (PPE)
Metallographers must take certain precautions to insure their personal safety. A laboratory coat is useful for protecting the operator’s clothing, and should be changed regularly and cleaned professionally. When handling caustics, a rubberized or plastic coated apron provides better protection. Gloves should be worn when handling bulk samples, working with hot material, or using hazardous solutions. Lightweight surgeons’ gloves are very popular, because the operator retains the ability to “feel.” Many metallographers wear these to protect their skin when mounting with epoxies, and polishing with oxide suspensions. When using these gloves with chemicals, always inspect for holes, as they are easily punctured. Thick rubber gloves are often used for handling specimens during macroetching, chemical polishing, pickling, etc. The gloves should always be checked first for small holes or cracks, Figure 3, because gloves can impart a false sense of security. The operator’s hands generally perspire when using rubber gloves and it is sometimes difficult to tell if the moisture is due solely to perspiration or to leakage. Safety glasses should be worn during processes that generate particulate matter. Goggles are appropriate for use with chemicals, and a chemical face shield is recommended when handling large quantities of hazardous liquids. The appropriate PPE will be specified on the MSDS for most laboratory chemicals and products.
The writer has seen metallography laboratories where the staff was forced to wear disposable rubber gloves all of the time when in the lab. While this practice sounds good, the staff can easily start to act as if they are “bullet proof” and do dumb things. One basic problem is that if the outside of the gloves are wet, you cannot feel that. Sometimes people get chemicals on the outside of the gloves and then transfer those chemicals unknowingly to other surfaces where someone might place their arm or elbow and get injured. The writer has seen microscope focusing controls become badly rusted from moisture on the outside of gloves. The 100% glove usage approach does have inherent problems and is not the answer to improving lab safety.
Chemicals, Storage and Handling
 |
| Figure 2. An eye wash unit should be in all laboratories working with potentially dangerous chemicals. |
Many of the chemicals used in metallography are toxic, corrosive, flammable, or potentially explosive. Therefore, whenever possible, purchase only small quantities that are likely to be used within a reasonably short time. Flammable solvents should be stored in fireproof steel cabinets. Acids and bases should be stored separately, again in fireproof steel cabinets. Strong oxidants must not be stored along with acids, bases or flammable solvents.
Reagent-grade chemicals or solvents of highest purity are recommended. Although more expensive, the amounts used are small and the gain in safety and reliability compensates for the cost difference. Chemicals may deteriorate during storage, another reason to purchase reasonably small quantities. Exposure to light can accelerate deterioration of some chemicals. Hence, they should be stored in a closed metal cabinet.
Etchants
Some etchants can be made as stock solutions and used over time, while others must be mixed fresh and may have a short useful life. For those that can be made up in quantity and stored, it is best to put them in a dark colored bottle, or store them in a closed cabinet. Many laboratories mix commonly used reagents in quantities of 250 to 1000 mL and then store them as stock reagents (clearly label the bottle!). Many reagents can be safely handled in this manner. It is best to store only those reagents that are used regularly. Glass-stoppered bottles are often used as stock reagent bottles. If these bottles are opened regularly, the stopper will not become “frozen”. However, if they are used infrequently, a frozen stopper often results. Holding the neck of the bottle under a stream of hot water will usually loosen them. If thermal expansion does not free the stopper, the stopper can be gently tapped with a piece of wood. Glass bottles with plastic screw-on tops can be used so long as the solution does not attack the plastic. These bottles are useful for holding solutions, such as nital, that can build up gas pressure within a tightly stoppered bottle. Nital with ≥5% nitric acid in ethanol should never be stored in a tightly closed glass bottle as pressure can build up and cause an explosion. The safe upper limit of nitric acid in methanol is higher, but the use of methanol should be restricted as much as possible as it is a cumulative poison to humans. Tightly stoppered bottles of nital and some other solutions have exploded as the result of pressure buildup. Be certain that the reagent is safe to store and store only small quantities. All bottles should be clearly labeled. Polyethylene bottles are required for etchants containing hydrofluoric acid, which attacks glass.
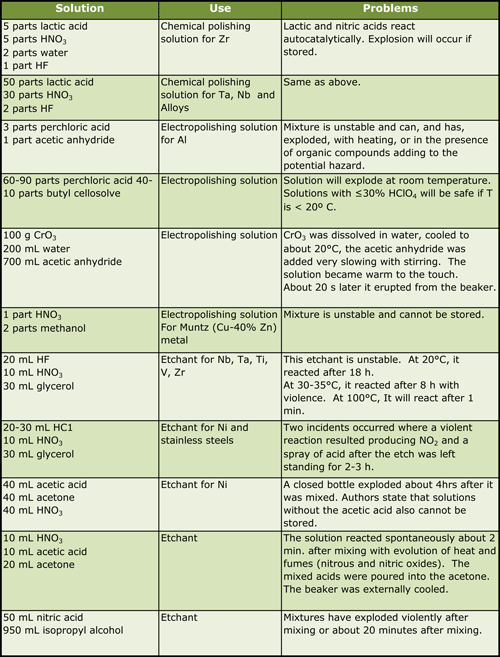 |
| Table 2. Chemical and Electrolytic Polishing Solutions and Etchants Known to be Dangerous |
Most recipes for etchants or electrolytes list the ingredients by weight if they are solids and by volume if they are liquids. In a few cases, all amounts are given in weight percentages. In many cases, but not all, precise control of reagent composition is not extremely critical. An ordinary laboratory balance provides adequate weighing accuracy, while graduated cylinders provide acceptable accuracy for volumetric measurements. These devices should be cleaned after use to prevent accidents to the next user. For weight measurements, a clean piece of filter paper, or a cup, should be placed on the balance pan to hold the chemical, to protect the pan surface, and to facilitate transfer to the mixing beaker. A large graduated beaker is usually employed for mixing solutions. When using HF, all containers should be made of polyethylene.
With some etchants, the mixing order is important, especially when dangerous chemicals are used. When water is specified, distilled water should always be used, because most tap water contains minerals or may be chlorinated or fluorinated. Tap water can produce poor results or unexpected problems. Cold water should always be used, never warm or hot water, which can cause a reaction to become violent. In mixing, one should start with the solvents, such as water or alcohol; then dissolve the specified salts. A magnetic stirring device is of great value, as shown in Fig. 4. Then, the dangerous chemicals, such as acids, should be added carefully and slowly while the solution is being stirred. Whenever sulfuric acid (H2SO4) is specified, it should be added last. It should be added slowly, while stirring, and it should be cooled, if necessary, to minimize heating. Never just pour one liquid into another, as shown in Fig. 5. If sulfuric acid is added to water without stirring, it can collect at the bottom of the beaker and enough local heating can occur to throw the contents out of the beaker.
The literature contains references to a great many formulas for etchants, chemical polishes, and electrolytes that are potentially dangerous or extremely dangerous. Few of these references contain comments regarding safe handling procedures or potential hazards. Fortunately, metallographic applications involve small quantities of these solutions, and accidents do not usually produce catastrophic results. However, even with small solution volumes considerable damage can be, and has been, done. Table 2 lists examples from the literature (1-7) of chemical polishing solutions, electrolytic polishing solutions and etchants that have been involved in accidents. Table 3 lists a number of commonly used chemicals and incompatible chemicals.
Solvents
Numerous organic solvents are used for cleaning or are ingredients in chemical or electrolytic polishing solutions or etchants, in which they are used to control ionization or the speed and mode of attack. Commonly employed solvents include water, acetone, ethyl ether, ethylene glycol, glycerol (glycerin), kerosene, petroleum ether, trichloroethylene, butyl cellosolve, and alcohols, such as amyl alcohol, ethanol, methanol, and isopropyl alcohol. Most are flammable and their vapors can form explosive mixtures with air. They should be kept closed when not in use and should be stored in a cool place away from heat and open flames.
Acetone (CH3COCH3) is a colorless liquid with a fragrant mint-like odor. It is volatile and highly flammable. It is an irritant to the eyes and the mucous membranes. Its vapor is denser than air and can travel along the ground and can be ignited at a distance. Acetone can form explosive peroxides on contact with strong oxidizers such as acetic acid, nitric acid and hydrogen peroxide. It is an irritant to the eyes and respiratory tract, and will cause the skin to dry and crack. It is mainly used to clean specimens before mounting.
Butyl cellosolve (HOCH2CH2OC4H9), or ethylene glycol monobutyl ether, is a colorless liquid with a rancid odor that is used in electropolishing solutions. It is combustible and may form explosive peroxides. It is toxic in contact with the skin, can be absorbed through the skin, can cause serious damage to the eyes, and irritation to the skin, and respiratory tract.
Carbitol (C2H5OCH2CH2OCH2CH2OH), or diethylene glycol monoethyl ether, is a colorless, viscous solvent that is compatible with water and is used in electropolishing solutions. It irritates the skin, eyes, mucous membranes, and upper respiratory tract, and is harmful if inhaled or swallowed.
Ethylene glycol (HOCH2CH2OH) is a colorless, hygroscopic liquid with a sweet taste (but do not swallow as it is poisonous) that reacts with strong oxidants and strong bases. It is slightly flammable. The substance irritates the eyes, skin, and the respiratory tract.
Glycerol (glycerin) (CH2OHCHOHCH2OH) is a colorless or pale yellow, odorless, hygroscopic, syrupy liquid with a sweet, warm taste. It is relatively nontoxic and nonvolatile but can cause iritis (inflammation of the iris). It is a combustible and is a moderate fire hazard. Glycerol should never be used in anhydrous solutions containing nitric and sulfuric acids, because nitroglycerin can form. Glycerol should not be used with strong oxidizing agents, such as chromium trioxide and potassium permanganate, as an explosion may occur. Glycerol is often added to aqua regia to make glyceregia. This mixture decomposes readily and should be discarded immediately after use. This etchant should not be allowed to stand for more than about 15-20 min. after mixing.
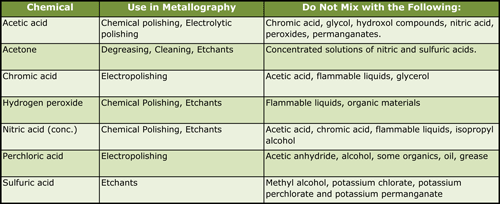 |
| Table 3. Some Incompatible Chemicals |
Kerosene is occasionally employed in grinding samples and with diamond paste as a lubricant. Only the deodorized form should be used. It is flammable, but the vapors do not readily explode. Contact defattens the skin can cause dermatitis, irritation or infections.
Trichloroethylene (CHCl:CCl2) is a stable, colorless liquid with a chloroform-like odor. . Effective laboratory ventilation is necessary. At ambient temperatures it is nonflammable and nonexplosive, but becomes hazardous at higher temperatures. In the presence of strong alkalies, with which it can react, it can form explosive mixtures. In the presence of moisture, the substance can be decomposed by light to corrosive hydrochloric acid. It is probably carcinogenic to humans, and toxic when inhaled or ingested, which may cause acute poisoning.
Amyl alcohol (CH3(CH2)4OH), or 1-Pentanol, is a colorless liquid with a noxious odor. It is flammable and the vapors may form explosive mixtures at elevated temperatures. The substance reacts violently with strong oxidants and attacks alkaline metals. The fumes are irritating to the eyes, upper respiratory tract, and skin. The substance is toxic through ingestion, inhalation, or absorption through the skin.
Ethyl alcohol (CH3CH2OH), or ethanol, is a colorless, inoffensive solvent commonly used in metallography. Ethanol is miscible with water and rapidly absorbs up to 5% water from the air. The denatured version is less expensive and contains 5% absolute methanol and is suitable for any recipe requiring ethyl alcohol. It is a dangerous fire hazard, and its vapors are irritating to the eyes and upper respiratory tract. High concentrations of its vapor can produce intoxication. Because ethanol is completely burned in the body, it is not a cumulative poison like methanol.
Methyl alcohol (CH3OH), or methanol, is an excellent, non-hygroscopic solvent, but it is a cumulative poison. Ingestion, inhalation or absorption through the skin in toxic levels can damage the central nervous system, kidneys, liver, heart, and other organs. Blindness has resulted from severe poisoning. It is particularly dangerous because repeated low-level exposures can also cause acute poisoning as a result of accumulation. Thus, whenever possible, ethanol should be used. When using methanol, always work under a ventilation hood. Mixtures of methanol and sulfuric acid can form dimethyl sulfate, which is extremely toxic. Solutions of methanol and nitric acid are more stable than mixtures of nitric acid and higher alcohols.
Isopropyl alcohol [CH3CH(OH)CH3], also known as 2-propanol, is a clear, colorless liquid that, like ethanol, does not accumulate in the body, although it does have a strong narcotic effect. It is a flammable liquid and is a dangerous fire hazard. Metallographers have used it as a substitute for ethanol but isopropyl alcohol has quite different characteristics and should not be used. Fatal injuries and explosions have been reported due to its use.
Acids
 |
| Figure 3. This glove will not protect you! Always inspect personal protective equipment before you put it on. |
Inorganic and organic acids are common constituents in chemical and electrolytic polishing solutions and in etchants. The inorganic, or mineral acids, including the very familiar acids such as hydrochloric, nitric, perchloric, phosphoric, and sulfuric, are highly corrosive and poisonous. They should be stored in a cool, well-ventilated location away from potential fire hazards and, of course, away from open flames. They should not be stored in a location that receives direct sunlight. When the pure acids contact metals, most liberate hydrogen gas – a fire and explosion hazard. The organic acids are naturally occurring substances in sour milk, fruits, and plants and include the following acids: acetic, lactic, citric, oxalic, and tartaric.
Hydrochloric acid (HCl), commonly used in metallography, is a colorless gas or fuming liquid with a sharp, choking odor. It is very dangerous to the eyes and irritating to the nose and throat. It attacks the skin strongly causing severe burns.
Nitric acid (HNO3), also commonly used in metallography, is a colorless or yellowish fuming liquid, highly toxic and dangerous to the eyes. If it contacts organic material or other easily oxidizable materials, it can cause fires and possibly explosions. When it reacts with other materials, toxic oxides of nitrogen are produced. The oxides, which vary with the conditions, include nitrous acid, nitrogen dioxide, nitric oxide, nitrous oxide, and hydroxylamine. A commonly encountered problem involves pouring nitric acid into a graduated cylinder that contains some methanol or ethanol from prior use. The brown fumes given off are quite harmful. Mixtures of nitric acid and alcohols higher than ethanol should not be stored. Mixtures of concentrated nitric and sulfuric acids are extremely dangerous, while strong mixtures of nitric acid and glycerin or glycols can be explosive. Aqua regia, a mixture of one part nitric acid and two to four parts hydrochloric acid, forms several products including nitrosyl chloride, an exceptionally toxic gas. Aqua regia is a popular etchant but must be used with extreme care under a hood. Never turn your back on aqua regia.
Ethanol with additions of up to 3 % nitric acid (nital) can be safely mixed and stored in small quantities. Higher concentrations result in pressure buildup in tightly stoppered bottles. Explosions of 5% nitric acid in ethanol have occurred as a result of failure to relieve the pressure. If higher concentrations are desired, they can be mixed daily, placed in an open dish, and used safely. Discard the etchant at the end of the day. Mixtures of methanol with up to 5% nitric acid are safe to use and store in small quantities. Mixtures of methanol with more than 5% nitric acid if heated are subject to violent decomposition. Mixtures of 33% nitric acid in methanol have decomposed suddenly and violently. Due to the extreme toxicity of methanol fumes over time, whose effects are not apparent until it is too late, methanol use should be eliminated as much as possible.
Never add nitric acid to isopropyl alcohol. Anderson (1) reported that a liter bottle of 5% nitric acid in isopropyl alcohol was mixed and placed in a cabinet. Although this had been done many times in the past without problems, twenty minutes later the bottle exploded destroying the cabinet, other stored bottles, and throwing debris up to 20 feet away. Anderson (1) also reported that a metallographer was pouring a freshly mixed liter of 5 % nitric acid in isopropyl alcohol into another bottle when it exploded. The person died within three hours without being able to tell anyone what happened. As with the other explosion, this same procedure had been performed many times previously without mishap. Anderson recommends avoiding use of isopropyl alcohol completely. This is wise advice.
Most metallographers consider nital to be very safe to use, and indeed it generally is. However, even with such an apparently safe solution, one can have accidents. One such accident occurred when an employee, not a skilled metallographer, was replenishing a stock of 3% nitric acid in ethanol using a procedure that he had claimed to have performed previously (he was not taught the safe way to mix nital as it was a union chemist’s job to mix nital; i.e., not his job). The worker began by adding the desired volume of concentrated nitric acid into the container that contained a small residual amount of stale nital. To his surprise, the contents began “boiling” and spewing out of the container along with dense, brown fumes. The acid splashed the worker, resulting in burns on his forehead, face and eyes. The small amount of aged nitric acid solution (the concentration may have been increased due to evaporation of the alcohol), present in the container when the fresh acid was added, created a dangerous chemical reaction. An experiment also showed that a similar reaction can occur when nitric acid is poured into a graduated cylinder containing only remnants of ethanol or methanol. If the employee had emptied and washed out the container first, mixed the ethanol and acid in a beaker and then poured it into the container, the accident would have been avoided.
Sulfuric acid (H2SO4) is a colorless, oily liquid that is highly corrosive, a strong oxidizing agent, and dangerously reactive. It reacts violently with bases and is corrosive to most metals forming flammable/explosive hydrogen gas. It reacts violently with water and organic materials with evolution of heat. Upon heating, toxic sulfur oxides are formed. One should add sulfuric acid very slowly to water with constant stirring. If added without stirring, it will produce a pocket of steam in the bottom of the vessel, throwing the contents out of the vessel. Concentrated sulfuric acid can cause severe, deep burns on contact with the skin, and permanent vision loss on contact with the eyes. Tissue is destroyed by the acid’s dehydrating action. Lungs may be affected by long-term or chronic exposure to its aerosol. Skin lesions, tooth erosion, and conjunctivitis are other long-term effects.
Hydrofluoric acid (HF) is a clear, colorless, fuming liquid or gas with a sharp, penetrating odor. It is very dangerous to the eyes, skin, and upper respiratory tract. The substance can be absorbed into the body by inhalation, through the skin and by ingestion. A harmful concentration of the gas in air can be reached quickly, making it very dangerous to handle. Exposure by ingestion, inhalation or contact, can be fatal. Undissociated HF poses a unique threat in that it can destroy soft tissues and result in decalcification of the bone. Moreover, the effects may be delayed. Laboratories where HF is used should stock an antidote kit to be used in case of exposure. Although it is a relatively weak mineral acid, HF will attack glass or silicon compounds, and should be measured, mixed, and stored in polyethylene vessels. HF reacts with many compounds, including metals, and will liberate explosive hydrogen gas.
Orthophosphoric acid (H3PO4), a colorless thick liquid or hygroscopic crystal, is a medium strong acid. It is corrosive to the skin, eyes and respiratory tract. Phosphoric acid decomposes on contact with alcohols, aldehydes, cyanides, sulfides, ketones, and can react with halogenated organic compounds forming organophosphorus nerve-gas type compounds that are extremely toxic. It reacts violently with bases, and will generate hydrogen gas when it reacts with metals.
Perchloric acid (HClO4) is a colorless, fuming, hygroscopic liquid. It is extremely unstable in concentrated form and may explode by shock or concussion when dry or drying, so commercially available perchloric acids come in concentrations of 65 to 72%. In this form, contact with perchloric acid will cause irritation and burns, while its fumes are highly irritating to the mucous membranes. Contact with organic, or other easily oxidized material, can form highly unstable perchlorates, which can ignite and cause explosions. Regular use of perchloric acid requires that the ventilation system must be specifically designed and maintained for perchloric acid. Special fume hoods with a waterfall-type fume washer will remove the perchlorate fumes before they can enter the exhaust system.
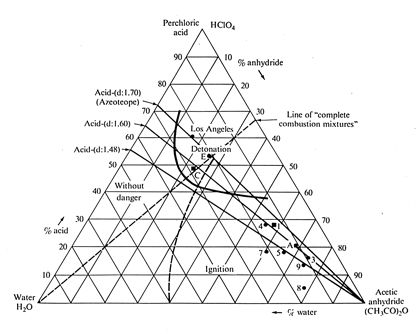
Perchloric acid is very useful in electropolishing solutions. However, never electropolish samples mounted in phenolic (“Bakelite”) or other plastics in perchloric acid solutions as explosions can result. The mixture of perchloric acid and acetic anhydride, which was developed by Jacquet, is difficult to prepare and highly explosive. Jacquet has reviewed the accidents involving perchloric acid and has described safety procedures (4). The worst accident occurred on February 20, 1947, in an electroplating factory in Los Angeles. In this accident 17 people were killed and 150 were injured (3). Medard, Jacquet, and Sartorius have prepared a ternary diagram showing safe compositions of perchloric acid, acetic anhydride, and water, Fig. 6. Anderson, however, states that accidents have still occurred with solutions in the “safe” region of this diagram (1). Thus, electropolishing solutions composed of perchloric acid and acetic anhydride are not recommended. Indeed, many companies forbid the use of such mixtures, and some cities have banned their use. Electropolishing solutions of perchloric acid and alcohol, with or without organic additions, and mixtures of perchloric acid and glacial acetic acid are safe to use. Nevertheless, in using these “safe” mixtures, one should follow the formula instructions carefully, mix only small quantities, keep the temperature under control, and avoid evaporation. These solutions should not be stored.
Mixtures of acetic acid and 5-10% perchloric acid have been commonly used to electropolish iron-based alloys and are reasonably safe. Do not use these solutions to electropolish bismuth, arsenic or tin, as explosions have occurred. Anderson suggests that arsenic, antimony, and tin may also be incompatible with perchloric electrolytes (1). Do not store these electrolytes for more than a few days. Discard them when they become colored by dissolved metallic ions (from electropolishing). Always keep these solutions cool; increasing the temperature increases the oxidizing power of perchloric acid.
Comas et al. have studied the hazards associated with mixtures consisting of butyl cellosolve and from 10 to 95% of 70% perchloric acid (5). Mixtures with 60 to 90% acid were explosive at room temperature. Acid concentrations of 30% or less were inflammable but were judged to be safe to use as long as the operating temperature does not exceed 20°C.
Acetic acid (CH3COOH) is a clear, colorless liquid with a pungent odor. It is a weak acid that reacts with strong oxidizers, bases and metals. It is flammable and is not easily ignited, although when heated, it releases vapors that can be ignited and can travel some distance to an ignition source. Contact with the skin results in serious burns. Inhalation of the fumes irritates the mucous membranes. Anderson states that acetic acid is a good solvent for nitric acid and that a 50% solution can be prepared, but not stored, without danger (1). Sax, however, states that mixtures of nitric and acetic acids are dangerous (6).
Acetic anhydride [(CH3CO)2O], or acetic oxide, is a colorless liquid with a very strong acetic odor. It can cause irritation and severe burns to the skin and eyes. Acetic anhydride decomposes on heating producing toxic fumes. It reacts violently with boiling water, steam, strong oxidants (specifically sulfuric acid), alcohols, amines, strong bases, and others. It attacks metals, and is very corrosive, especially in presence of water or moisture. It is extremely flammable and should be avoided. The electrolytic polishing mixtures of acetic anhydride and perchloric acid (4-to-1 to 2-to-1 mixtures) developed by Jacquet, as mentioned above, are exceptionally dangerous and should never be used. Dawkins (7) reported an accident involving a mixture of chromium trioxide and acetic anhydride that had been used for electropolishing (Table 2).
Citric acid [C3H4(OH)(COOH)3•H2O] comes as colorless, odorless crystals that are water-soluble. It is an irritant to the skin, eyes and respiratory tract, with no unusual problems are encountered except for occasional allergic reactions.
Lactic acid (CH3CHOHCOOH) is a yellow or colorless thick liquid. It is damaging to the eyes.
Oxalic acid (COOHCOOH•2H2O) comes as transparent, colorless crystals. It is poisonous if ingested and irritating to the upper respiratory tract and digestive system if inhaled. Skin contact produces caustic action and will discolor and embrittle the fingernails. It is not compatible with nitric acid, as it reacts violently with strong oxidants. It can also form explosive compounds due to reacts with silver.
Picric acid [(NO2)3C6H2OH], or 2,4,6-Trinitrophenol, comes as yellow crystals that are wet with 10 to35% water. When picric acid is dry, it will explode if a spark touches it. It is toxic and stains the skin. It is incompatible with all oxidizable substances. Picrates, which are metal salts of picric acid, are explosive. When picrates are dry, they can detonate readily, possibly spontaneously. Purchase in small quantities, keep it moist, and store it in a safe, cool place. If it starts to dry out, add a small amount of water to keep it moist. The maximum solubility of picric acid in water and in ethanol is about 1.3 and 8 g per 100 mL, respectively. Picral can be stored safely. During use, the solution should not be allowed to dry out. The etching residue should be discarded at the end of the day to avoid potential explosions. Picric acid use has been outlawed by some companies and in some states. Metallography laboratory accidents attributed to picric acid, or its use in 4% picral or Vilella’s reagent, have been almost non-existent, especially when compared to the supposedly very safe nital. In 47 years of metallography, the writer has never heard a report of an accident from picric acid, 4% picral, or Vilella’s reagent, but has heard of 4 accidents using the supposedly much safer nital. The writer has been contacted several times over the years by people at schools where a student has discovered an old bottle of picric acid in a cabinet. Despite having dried out, no explosion occurred as sparks were not in contact with the dried picric acid. The simple solution is to fill a sink or bucket with water, open the bottle under water and allow water to enter, and then reclose the bottle.
Bases
Bases, such as ammonium hydroxide (NH4OH), potassium hydroxide (KOH), and sodium hydroxide (NaOH), are commonly used in metallography, chiefly in etchants.
Ammonium hydroxide is a colorless liquid with a strong, obnoxious odor. Solutions are extremely corrosive and irritating to the skin, eyes, and mucous membranes. It reacts exothermically with sulfuric acid and other strong mineral acids, producing boiling solutions.
Sodium and potassium hydroxides are strong bases, available as white deliquescent pellets that are soluble in water. They can rapidly absorb carbon dioxide and water from the air. Solutions stored in flasks with ground stoppers may leak air and freeze the stoppers, making re-opening difficult. Dissolving NaOH or KOH in water will generate considerable heat. Do not dissolve either in hot water. Never pour water onto these hydroxides; always add the pellets slowly to the water. Alkali metal hydroxides react violently with acid, and are corrosive in moist air to metals like zinc, aluminum, tin and lead forming flammable/explosive hydrogen gas. They are very corrosive to skin, eyes and respiratory tract. Long-term exposure may lead to dermatitis. Potassium hydroxide is somewhat more corrosive than sodium hydroxide.
Other Chemicals Used by Metallographers
Hydrogen peroxide (H2O2) is available as a liquid in concentrations of either 3 or 30%. The 3% solution is reasonably safe to use, while the 30% solution is a very powerful oxidant whose effect on the skin is about as harmful as that produced by contact with sulfuric acid. Hydrogen peroxide by itself is not combustible, but if brought in contact with combustible materials, it can produce violent combustion. Hydrogen peroxide is very damaging to the eyes. Because release of oxygen can cause high pressures to develop within the container, the container caps are vented.
Bromine (Br2), a fuming reddish brown liquid with a pungent, suffocating odor, has been used in deep-etching solutions to reveal second-phases in Fe-based alloys. It is very corrosive, reacting violently with easily oxidized substances, including some metals. Bromine is a dangerous liquid that should only be handled by well-qualified personnel. Its vapors are extremely irritating to the eyes, skin, and mucous membranes. Skin contact produces deep, penetrating burns that are slow to heal. Contact with organic matter can cause fires.
Chromic acid (H2CrO4) is formed when chromium trioxide (CrO3) is dissolved in water. CrO3 is used in electropolishing solutions (see previous comment and Table 2 about explosive nature of mixtures with acetic anhydride). Dilute aqueous solutions are widely used for attack polishing. It is a powerful oxidant; always wear gloves when using it for attack polishing or use automatic devices and avoid contact potential. Chronic or long-term inhalation exposure may produce asthma-like reactions.
Potassium permanganate (KMnO4), a black crystalline powder, is a powerful oxidant used in etchants. It is a dangerous fire and explosion hazard, especially when in contact with organic materials. Ingestion produces serious damage. KMnO4 and sulfuric acid should never be mixed together because a violent explosion can result.
Potassium dichromate (K2Cr2O7), a bright orange crystalline powder, is another powerful oxidant that is also used in etchants. Contact can cause ulceration of the hands, severe damage to nasal tissue, or asthma and allergies with long-term exposure.
Cyanide compounds are occasionally used in metallographic applications. Potassium cyanide (KCN) and sodium cyanide (NaCN) are extremely dangerous and highly toxic. Exposure by eye or skin contact, or by ingestion, is fatal. NaCN and KCN vapors are intensely poisonous. They are particularly hazardous when brought in contact with acids or acid fumes because of liberation of hydrogen cyanide, which is extremely toxic and highly flammable. Potassium ferricyanide (K3Fe(CN)6), a ruby-red crystalline powder and an ingredient in Murakami-type reagents, is poisonous but stable and reasonably safe to use.
A number of nitrates, such as ferric nitrate [Fe(NO3)3•6H2O], lead nitrate [Pb(NO3)6], and silver nitrate (AgNO3), are sometimes used by metallographers. Since they are powerful oxidizers, they pose a dangerous fire hazard, especially when in contact with organic materials. They may evolve toxic fumes, such as oxides of nitrogen and lead, and are poisonous and corrosive to the eyes, skin and respiratory tract.
Conclusions
In general, the metallographic laboratory is a reasonably safe environment. However, depending on the materials being prepared, dangerous situations can arise. Some hazards, such as the preparation of radioactive or reactive metals, are quite obvious, while others are not. In the preceding discussion, some of the potential hazards that can be encountered are summarized; others undoubtedly exist that are not covered.
Most accidents can be prevented by simple common sense rules. It is best to assume that all metal dust and all chemicals are hazardous. Inhalation of dust and fumes, ingestion, or bodily contact should be avoided. Personal protective equipment is very useful, but it should not be used as a substitute for good, safe laboratory practices. The use of such equipment does not guarantee freedom from injury.
The metallographic literature contains many references to the use of dangerous materials, often without any mention of the dangers involved or safe handling procedures. This is unfortunate because the unwary may be injured. Many of us are tempted to experiment when a recommended procedure does not work as claimed. The development of electrolytes, chemical polishing agents, or etchants should be left to those who are fully versed in the potential dangers. Metallographic laboratories should have some of the referenced safety publications (see bibliography) readily available in the laboratory and these safety publications should be consulted when working with new or infrequently used materials.
REFERENCES
1. R. L. Anderson, “Safety in Metallographic Laboratory,” Westinghouse Research Laboratory Science Paper No. 65 – 1P30 – METLL – P2, March 29, 1965.
2. G. F. Vander Voort, Metallography: Principles and Practice, ASM International, Materials Park, OH., 1999, pp. 148-159.
3. R. C. Nester and G. F. Vander Voort, “Safety in the Metallographic Laboratory,” Standardization News, Vol. 20, May 1992, pp. 34-39.
4. P. A. Jacquet, “The Safe Use of Perchloric-Acetic Electropolishing Baths,” Met. Finish, Vol. 47, 1949, pp. 62-69.
5. S. M. Comas, R. Gonzalez Palacin and D. Vassallo, “Hazards Associated with Perchloric Acid-Butylcellosolve Polishing Solutions,” Metallography, Vol. 7, 1974, pp. 47-57.
6. N. I. Sax, Dangerous Properties of Industrial Materials, 5th ed., Van Nostrand Reinhold Co., Inc., N.Y., 1979.
7. A. E. Dawkins, “Chromic Acid-Acetic Anhydride ‘Explosion,’” J. Iron and Steel Institute, Vol. 182, 1956, p. 388.
Selected Bibliography of Books on Laboratory Safety
ASTM E 2014, Standard Guide on Metallographic Laboratory Safety.
Norman V. Steere (ed.), Handbook of Laboratory Safety, 2nd ed., CRC Press, Boca Raton, FL., 1971.
N. Proctor and J. Hughes, Chemical Hazards in the Workplace, J. B. Lippincott Co., Philadelphia, 1978.
N. I. Sax, Dangerous Properties of Industrial Materials, 5th ed., Van Nostrand Reinhold Co., N.Y., 1979.
L. Bretherick, Handbook of Reactive Chemical Hazards, 2nd ed., Butterworths, London, 1979.
F. A. Patty (ed.), Industrial Hygiene and Toxicology, Volume II – Toxicology, 3rd ed., Wiley-Interscience, N.Y., 1980.
A. A. Fuscaldo et al. (eds), Laboratory Safety: Theory & Practice, Academic Press, San Diego, CA. 1980.
Prudent Practices for Handling Hazardous Chemicals in Laboratories, National Academy Press, Washington, D.C., 1981.
Prudent Practices for Disposal of Chemicals from Laboratories, National Academy Press, Washington, D.C., 1983.
C. A. Kelsey and A. F. Gardner (eds.), Radiation Safety for Laboratory Technicians, Warren H. Green, Inc., St. Louis, MO., 1983.
S. B. Pal (ed.), Handbook of Laboratory Health & Safety Measures, Kluwer Academic, Norwell, MA., 1985.
L. J. Diberardinis et al., Guidelines for Laboratory Design: Health & Safety Considerations, J. Wiley & Sons, N.Y., 1987.
S. R. Rayburn, The Foundations of Laboratory Safety, Brock-Springer Series in Contemporary Bioscience, Springer-Verlag, N.Y., 1989.
N. Van Houten, Laboratory Safety Standards for Industry, Research Academe, Sci. Tech. Pubs., Lake Isabella, CA. 1990.
E. Gershey, A. Wilkerson and E. Party, Laboratory Safety in Practice, Van Nostrand Reinhold, N.Y., 1991.
R. J. Lewis, Sr. (ed.), Hawley’s Condensed Chemical Dictionary, 12th Ed., Van Nostrand Reinhold, New York, 1993.
A. Keith Furr (ed.), Handbook of Laboratory Safety, 5rd ed., CRC Press, Boca Raton, FL., 2000.
R. J. Alaimo (ed.), Handbook of Chemical Health and Safety, ACS Handbook, 2001.
R. Lewis, Sax’s Dangerous properties of Industrial Materials, 11th ed., J. Wiley & Sons, NY, 2004.
Prudent Practices in the Laboratory: Handling and Management of Chemical Hazards, Updated Version, National Academic Press, 2011
George Vander Voort has a background in physical, process and mechanical metallurgy and has been performing metallographic studies for 47 years. He is a long-time member of ASTM Committee E-4 on metallography and has published extensively in metallography and failure analysis. He regularly teaches MEI courses for ASM International and is now doing webinars. He is a consultant for Struers Inc. and will be teaching courses soon for them. He can be reached at 1-847-623-7648, EMAIL: georgevandervoort (at) yahoo.com and through his web site: www.georgevandervoort.com
To View a listing of all George’s articles please click here
Read George Vander Voort’s Biography