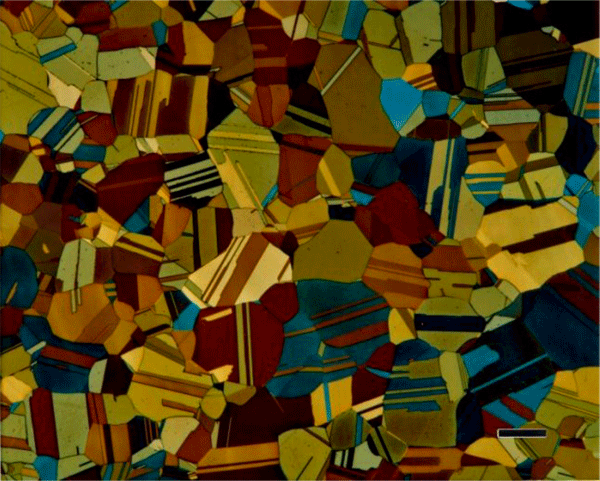Color has historically seen limited use in metallography, mainly due to the cost of film and prints and the difficulty and cost of reproducing images in publications. However, with the growth of digital imaging, capturing color images is much simpler and cheaper. Also, printing images in color is inexpensive for in-house reports, and can be distributed cheaply on CDs, although reproduction in journals is still expensive. Color does have many advantages over black and white.
Color has historically seen limited use in metallography, mainly due to the cost of film and prints and the difficulty and cost of reproducing images in publications. However, with the growth of digital imaging, capturing color images is much simpler and cheaper. Also, printing images in color is inexpensive for in-house reports, and can be distributed cheaply on CDs, although reproduction in journals is still expensive. Color does have many advantages over black and white.
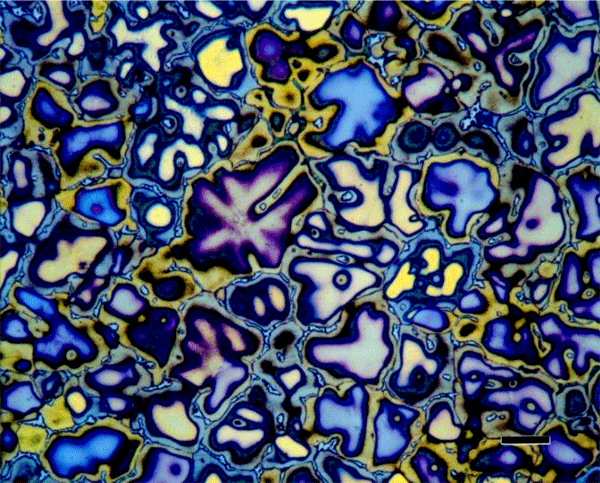
First, the human eye is sensitive to only about forty shades of gray from white to black, but is sensitive to a vast number of colors. Tint etchants reveal features in the microstructure that often cannot be revealed using standard black and white etchants. Color etchants are sensitive to crystallographic orientation and can reveal if the grains have a random or a preferred crystallographic texture. They are also very sensitive to variations in composition and residual deformation. Further, they are usually selective to certain phases and this is valuable in quantitative microscopy.
Introduction
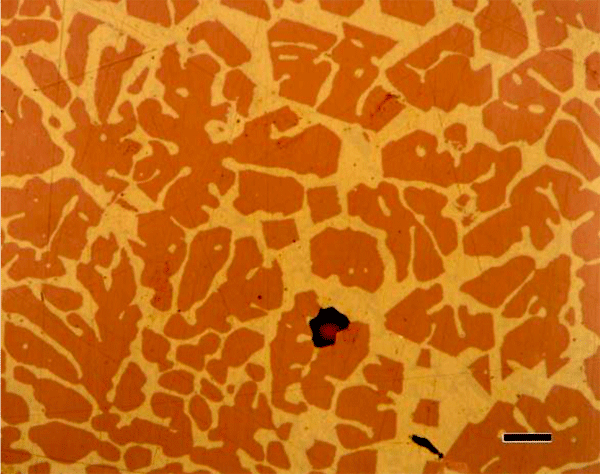
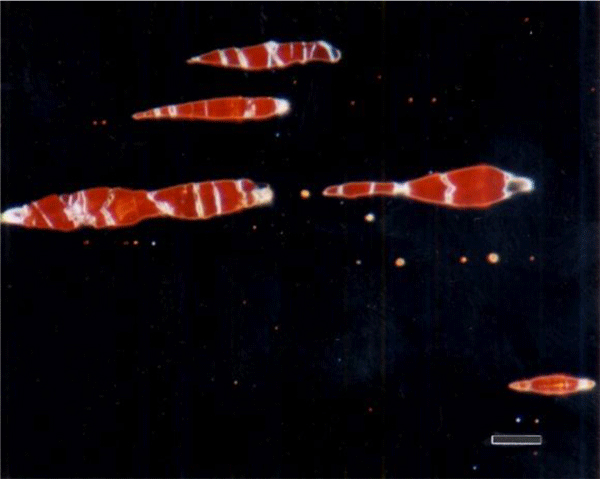
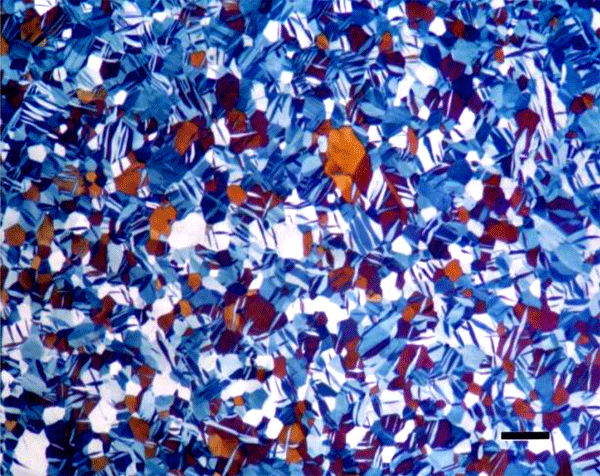
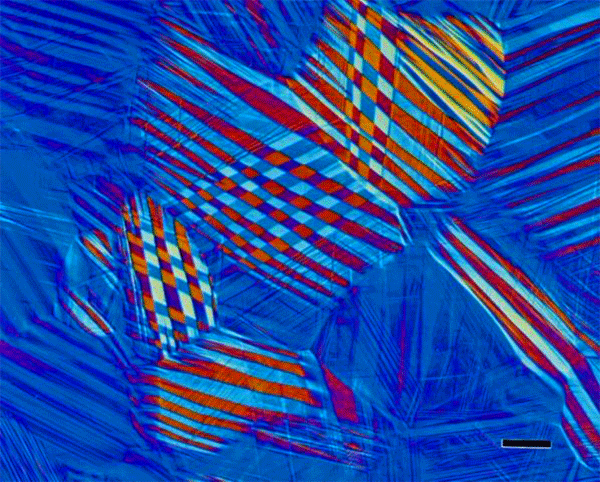
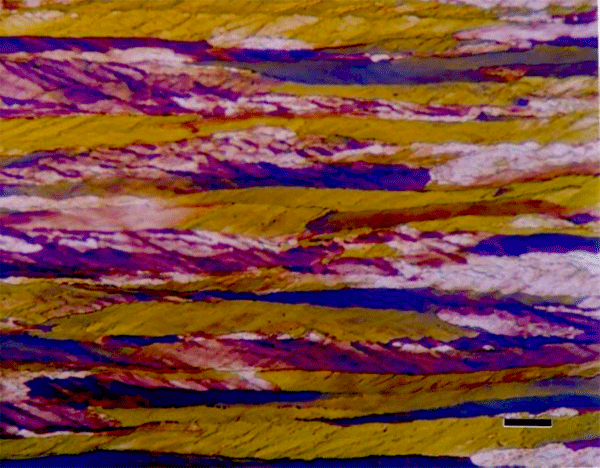
The use of color in metallography has a long history with color micrographs published over the past eighty-some years. Examples of natural color in metals are rare (Figure 1). Gold and copper exhibit yellow color under bright field illumination. Color can be produced using optical methods, as in dark field illumination (Figure 2), polarized light (Figure 3) and differential interference contrast illumination (Figure 4). The microstructure of metals with non-cubic crystal structures can be examined without etching using polarized light but color is not always observed. The specimen must be prepared completely free of residual damage for color to be observed, and even then, some non-cubic metals still exhibit little color. However, many metals and alloys can be etched with reagents that deposit an interference film on the surface that creates color in bright field illumination. If it is difficult to grow such a film to the point where the color response is excellent, the color can be enhanced by examination with polarized light, perhaps aided with a sensitive tint filter (also called a lambda plate or first-order red filter).
Anodizing
 |
|
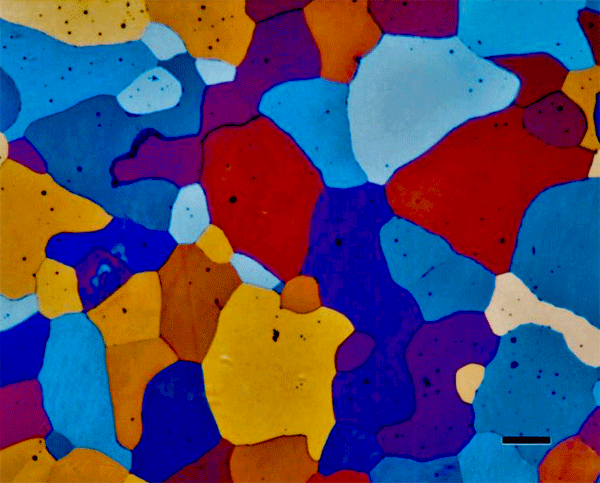
Figure 5. Super-pure aluminum anodized with Barker’s reagent (30 V dc, 2 minutes). The magnification bar is 200 µm long
|
There are a number of electrolytic etching reagents that can be used to produce color. Second-phase constituents can be colored and viewed with bright field. Anodizing aluminum specimens with Barker’s reagent, or similar solutions, does not produce an interference film, as color is not observed in bright field. This procedure produces fine etch pitting on the surface. The grain structure can be seen in black and white in polarized light, and in color if a sensitive tint plate is added. Figure 5 show an example of anodizing to reveal the grain structure of super-pure aluminum.
Color Etching
Many metals etched with standard reagents to reveal the grain boundaries often yield only a high percentage of the boundaries, rather than all of the boundaries. Color etchants, however, reveal the grain structure completely. In the case of metals with annealing twins, it can be very difficult to rate the grain size when a standard etchant reveals a portion of the grain and twin boundaries. In fact, it can be quite difficult to make a precise measurement of the grain size, even manually, with such a specimen, as distinguishing between grain and twin boundaries (the latter must be ignored in the measurement), is not simple. However, with a color etched microstructure it is relatively easy to separate grain from twin boundaries, at least manually. Further, the films grow as a function of crystal orientation. Therefore, one can detect any preferred crystallographic orientation by the narrowness of the color range present. If a wide range of colors is present in a random pattern, the crystal orientation is random. If a narrow range of colors is present in the grains, then a preferred orientation is present. Tint etch compositions are given at the end of the paper.
Specimen preparation must be better when using color methods than for black and white methods because the epitaxially grown films are sensitive to residual preparation-induced damage that was not removed. This level of preparation is required in image analysis work and can be easily obtained by a knowledgeable metallographer with the proper equipment. Electrolytic polishing is not required to get damage-free surfaces.
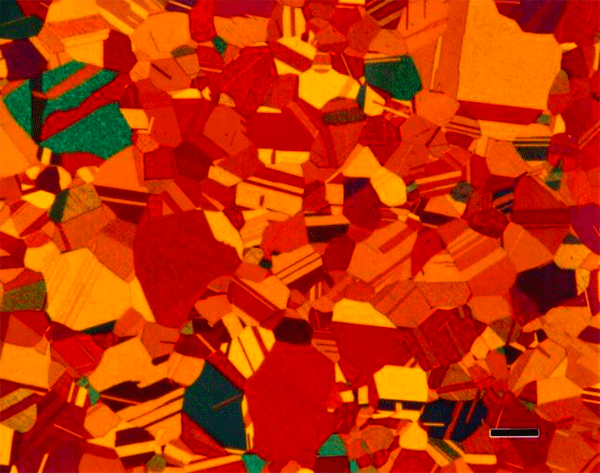
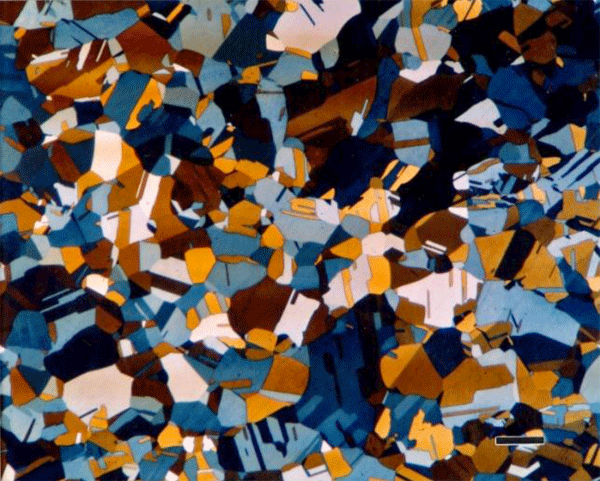
The most common tint etchants are those that deposit a sulfide-based interference film on the specimen. These are the best-known tint etches and usually the easiest to use. Klemm and Beraha have developed the most widely used sulfide-based tint etchants using sodium thiosulfate, Na2S2O3, and potassium metabisulfite, K2S2O5. Klemm’s I, II, III (Figures 6 and 7) and one of Beraha’s reagents utilize both ingredients (Figure 8) while Beraha recommends a range of HCl concentrations used with potassium metabisulfite (Figure 9) for etching a variety of iron-based alloys. These etchants can be used to color ferrite and martensite in cast iron, carbon and low-alloy steels. The HCl-based reagents vary widely in concentration and can be used to color the grain structures of stainless steels (Figure 10), Ni-based and Co-based alloys. Sodium metabisulfite has been used in a number of concentrations, from about 1 to 20 g per 100 mL water, and is a safe, reliable, useful color etch for irons and steels (Figure 11).
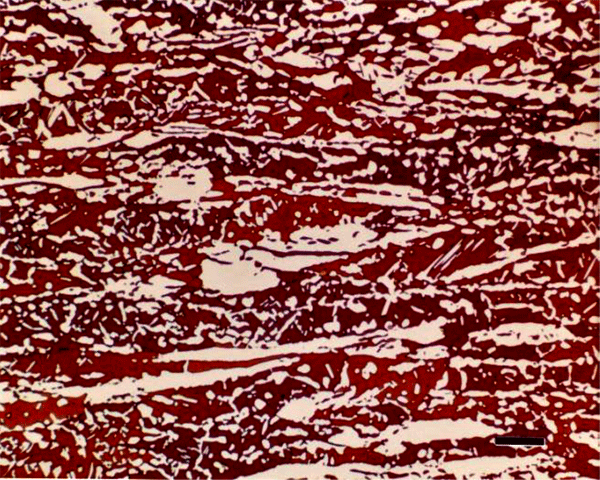
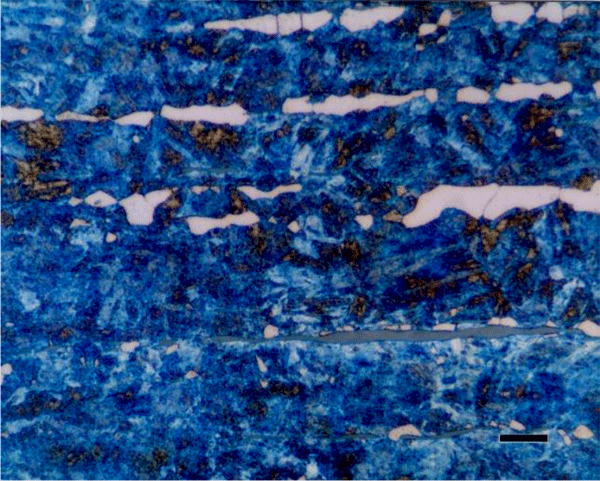
Beraha also developed etchants based upon sulfamic acid, a weak organic acid, that have not been used much, although they are quite useful, reliable and easy to employ. The sulfamic acid-based reagents are applicable to cast iron, low-carbon and alloy steels, tool steels, and martensitic stainless steels (Figure 12). Beraha also developed two rather specialized tint etches that deposit cadmium sulfide (Figure 13) or lead sulfide (Figure 14) films on the surfaces of steels and copper-based alloys. These two etchants are quite useful, although tedious to make. His CdS reagent is useful for carbon and alloy steels, tool steels, and ferritic, martensitic and precipitation hardenable stainless steels while the PbS reagent does an excellent job on copper-based alloys and can be used to color sulfides in steels white (the specimen is pre-etched with nital and the etch colors the darkened matrix, so that the white sulfides are visible).
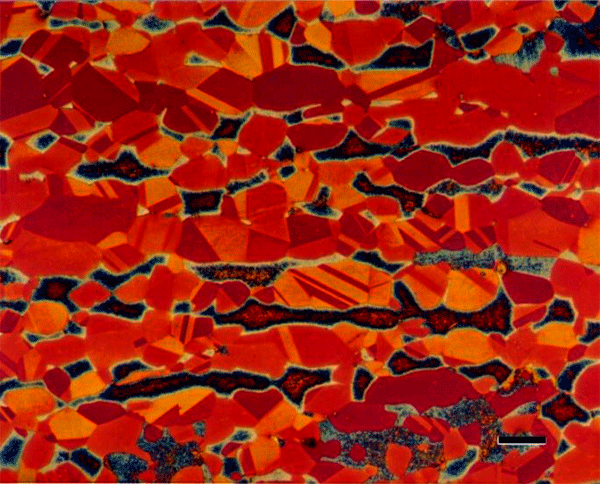
Beraha also developed two tint etchants that utilize molybdate ions in nitric acid. They color cementite in steels (Figure 15). He also developed tint etchants that deposit elemental selenium on the surface of steels (Figure 16), nickel-based alloys and copper-based alloys (Figure 17).
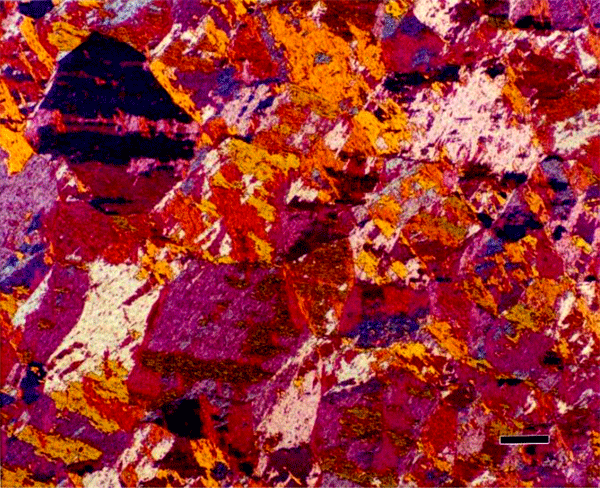
There are a number of other tint etchants that have been developed by a variety of metallographers. Lichtenegger and Blöch, for example, developed an unusual reagent that will color austenite (Figure 18) in duplex stainless steels, rather than ferrite (as nearly all others do).
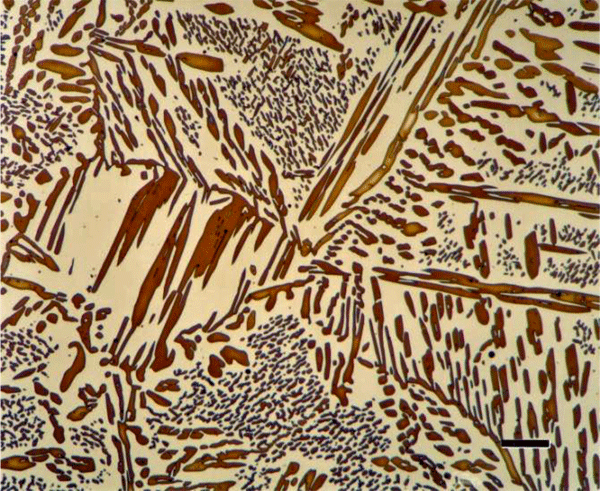
Weck developed a number of tint etchants, while utilizing many of them in her research. Several were developed to color aluminum (Figure 19) or titanium alloys (Figure 20). In each case, it is easier to develop good color with the cast alloys than with the wrought alloys. Two etchants have been found useful for coloring theta phase, AlCu2, in Al-Cu alloys; Lienard developed one of the easiest to use. Several color etchants have been developed for molybdenum (Figure 21) and for tungsten. Details on these etchants can be found in [1].
Conclusions
The examples shown have demonstrated the great value of color and tint etching for examining microstructures of metals. Solutions exist to develop color with most commercial alloy systems. The examples clearly demonstrate the value of these reagents in revealing the grain structure fully, even for the most difficult to etch specimens. Further, they are selective in nature that can be quite useful for quantitative metallographic studies. Tint etchants reveal segregation very clearly and either EDS or WDS can be performed on a tint-etched surface without any problems from the interference surface layer.
Appendix
- Etch Compositions
- Klemm’s I: 50 mL stock solution, 1 g K2S2O5 (stock solution is water saturated with Na2S2O3
- Klemm’s III: 5 mL stock solution, 45 mL water, 20 g K2S2O5 (stock solution as for Klemm’s I)
- Beraha’s 10/3 reagent: 10g Na2S2O3, 3g K2S2O5 and 100 mL water
- Beraha’s BI: 100 mL stock solution (1000 mL water, 200 mL HCl, 24 g NH4FHF) plus 0.1 – 0.2 g K2S2O5 for martensitic stainless steel and 0.3 – 0.6 g K2S2O5 for austenitic and ferritic stainless steels.
- Beraha’s sulfamic acid reagent No. III: 100 mL water, 3 g K2S2O5, 2 g NH2SO3H (two other similar compositions were published) for carbon and alloy steels.
- Beraha’s sulfamic acid reagent No. IV: 100 mL water, 3 g K2S2O5, 1 g NH2SO3H, 0.5 – 1 g NH4FHF for high-Cr tool steels and martensitic stainless steels.
- Beraha’s CdS and PbS reagents: CdS stock solution: 1000 mL water, 240 g Na2S2O3 • 5H2O, 20-25 g cadmium chloride (or cadmium acetate), 30 g citric acid; PbS stock solution: 1000 mL water, 240 g Na2S2O3 • 5H2O, 30 g citric acid, 24 g lead acetate.
- Mix each in order given. Age solutions in a dark bottle, in darkness, for 24 hours before use. To use the CdS reagent, filter excess precipitates from about 100 mL of solution. CdS regent will color microstructure of a wide range of irons and steels in 20 to 90 s. To use the PbS reagent, do not filter excess precipitates when you pour about 100 mL of the stock solution for use. The PbS reagent will color the microstructure of most copper-based alloys. It will also etch steel microstructures.
- Beraha’s sodium molybdate reagent: Stock Solution: 1000 mL water, 10 g Na2MoO4 • 2H2O. Pour off about 100 mL of the stock solution and add HNO3 to bring the pH to 2.5 – 3.0. For steels, add small amounts of NH4FHF to control coloration (none for cast iron). Colors cementite.
- Beraha’s selenic acid reagent for cast iron: 100 mL ethanol, 2 mL HCl, 1 mL selenic acid.
- Beraha’s selenic acid reagent for Cu alloys: 300 mL ethanol, 2 mL HCl, 0.5-1 mL selenic acid
- Lichtenegger and Blöch LB1 reagent: of 20 g of ammonium bifluoride, NH4FHF, and 0.5 g potassium metabisulfite, K2S2O5, dissolved in 100 mL water (use hot water). Etch at 25-30 ºC.
- Weck’s reagent for Al: 100 mL water, 4 g KMnO4 and 1 g NaOH
- Modified Weck’s reagent for Ti: 100 mL water, 25 mL ethanol and 2 g ammonium bifluoride. The original formula specified 50 mL of ethanol, but that produces etch artifacts.
- ORNL tint etch for Mo: 70 mL water, 10 mL sulfuric acid and 20 mL hydrogen peroxide (30% conc.). The specimen is immersed for 2-3 minutes. Swab etching produces a flat etched microstructure (no color).
George Vander Voort has a background in physical, process and mechanical metallurgy and has been performing metallographic studies for 43 years. He is a long-time member of ASTM Committee E-4 on metallography and has published extensively in metallography and failure analysis. He regularly teaches MEI courses for ASM International and is now doing webinars. He is a consultant for Struers Inc. and teaches courses for them. He can be reached at 847-623-7648, georgevandervoort@yahoo.com and through his web site: www.georgevandervoort.com
(Courtesy of the Microscopy Society of America – www.microscopy.org) Original Published by Microscopy Today, November 2005 – Volume 13, Number 6 – www.microscopy-today.com
To View a listing of all George’s articles please click here
Read George Vander Voort‘s Biography
Reference
[1]. G. F. Vander Voort, Metallography: Principles and Practice, McGraw-Hill Book Co., NY, 1984 and ASM International, Materials Park, Ohio, 1999.