Manufacturers of materials, components, and machines for spacecraft and satellites deployed in space must vigorously test them prior to putting them into service. For example, linear actuator mechanisms on satellites have failed to function properly (extend or retract) because of a loss of tolerance due to the conversion of retained austenite to martensite and subsequent growth of the part due to volume expansion. Had this test not been performed in a simulation chamber at -62ºC (-80ºF) here on Earth, a solar array or communications antenna would not have deployed when the satellite was in orbit and its mission would have been compromised.
In order to ensure thermal and vacuum readiness of these systems prior to lift off, they must be subjected to the extreme vacuum and temperature of space to ensure they can withstand and perform under these harsh conditions without failure. Space simulation (aka space test) chambers are used to perform this testing. The challenging conditions encountered in space and the development of the space simulation chamber are the focus of our discussion.
What is the Vacuum of Space?
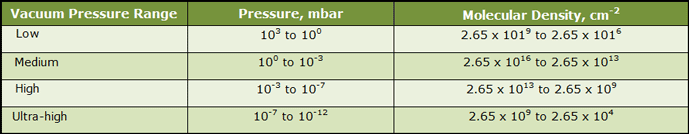
Space is almost a perfect vacuum. It is not perfect because there are molecules present and the pressure is not zero. In space, the pressure is actually in the range of 10-12 mbar, or one billionth the pressure here on Earth. This is comparable to the lowest vacuum achievable on Earth with an ultra-high vacuum system. The concept of ultra-high vacuum can be difficult to comprehend because of the extremities of pressure involved. It is easy to understand how an everyday vacuum cleaner produces enough suction to reduce the air pressure at its inlet to 20% below atmospheric pressure (which also happens to be the air pressure at 2,000 meters (6,560 feet) above sea level). These pressures are intuitive. However, as one travels higher and higher in altitude and leaves earth’s atmosphere entirely, the pressure drops to one billionth (10−9) that of atmospheric pressure.
On Earth, the normal atmospheric pressure of the air we feel on our bodies is caused by the weight of the air above us being pulled towards earth by gravity. Air is heavier than we instinctively realize, with a weight of 1.22 kilograms per cubic meter (0.08 pounds per cubic foot).
Considering the height of air in the atmosphere above us, the concept of atmospheric pressure is more understandable. Atmospheric pressure at the Earth’s surface is 10,330 kilograms per square meter (21,168 pounds per square foot, or 14.7 pounds per square inch). This pressure of the air on the surface of our skin is caused by the weight of the column of air directly above us, going all the way out to the edge of space. People that suffer from arthritis or other joint ailments often complain of increased pain when the weather changes. This is thought to be due to the change in barometric (atmospheric) pressure on our bodies. It isn’t unusual for the barometric pressure to change by 16.5 mbar (0.5 inches of mercury) from one day to the next. This may not seem like much but equates to a change of 17 kilograms of force on each square meter (35.4 pounds on each square foot) of the body. When the air pressure drops too suddenly for the fluid inside a knee joint to equalize, the unequal pressure between the inside and outside of the joint causes the soft tissue and fluids around the joint to press outward, causing irritation and pain.
In space, however, where there is no gravity to pull the gas molecules together, they have no weight, and pressure caused by gravitational forces does not exist. The reason for this lack of molecules in space is that the gravitational pull of large bodies such as planets and stars attracts most loose gas molecules and gather them together. With no gravity present in space, and so few gas molecules in a given volume, the primary source of pressure on a spacecraft is the collisions of the gas molecules with the wall of the craft as they move about due to molecular motion. The lack of gravity and the very small number of molecules in space explains the extremely low pressure in space.
Another way to understand the vacuum of space is to consider the number of gas molecules that reside in a given volume (Table 1). There are roughly 2.65 x 1019 or 26,500,000,000,000,000,000 molecules in a cubic centimeter of gas at a pressure of 1,000 mbar (atmospheric pressure at sea level). To put this into perspective, if these molecules were grains of sand, this number of them, tightly packed, would fill up the entire volume of the Empire State Building three times over.
At increasing distance from Earth, the pressure becomes lower and lower and the molecules spread out further and further until at ultra-high vacuum (10-12 mbar), there are only 2.65 x 104 or 26,500 molecules per cubic centimeter. At this density, there is only one molecule roughly every 0.33 mm (0.012 inches) in space. Since the diameter of each gas molecule is so much less than this (1 x 10-7 or .0000001 mm for hydrogen, for example), there is a great deal of space between them. If hydrogen molecules were grains of sand, in the vacuum of space they would be 159 meters (521 feet) apart.
Extreme Temperatures in Space
The extremely low pressure in space is not the only challenge faced by spacecraft, thrusters, sensors, and other components used in space. This equipment must also withstand extremes of temperature. Theoretical models can only provide us with so much information. In order to properly test these components and verify proper operation prior to blast off, space test chambers must not only generate ultra-high vacuum but must also simulate the extreme temperatures encountered in space.
In order to understand the temperature extremes in space it is helpful to review the basics of heat transfer. Temperature is a measure of the translational kinetic energy of the molecules in a body, where the hotter an object is, the more kinetic energy its molecules have. Any time two bodies have different temperatures, heat transfer or heat exchange occurs between them. The transfer always occurs from the body with the higher temperature to the body with the lower temperature, and can take place in three ways; conduction, convection, and radiation (Fig. 2).
Heat conduction is caused by the transfer of heat energy between objects in physical contact with one another. An example is an egg cooking in a skillet, where heat is transferred by conduction from the skillet to the egg. Conduction is not a factor for man-made objects in space because they are not in direct contact with any heat source. The second method of heat transfer, convection, relies on a gas or liquid to transfer heat to, or from, an object. An example is how a fan makes you feel cool or how a hair dryer makes your skin feel hot. Since in space there is no gas or liquid to transfer heat, convection does not play a role in the temperature of objects. Radiation is the third method of heat transfer and occurs between two bodies, which do not touch and require no physical medium (such as a liquid or gas) between them. One example is electromagnetic radiation. An example is how heat from the sun feels warm or how a toaster turns bread into toast. Radiant heat energy is carried by photons, does not require an intermediate gas or liquid, and occurs readily in the vacuum of space.
The primary source of heat energy in our solar system is radiant heat from the sun. Since this energy travels only in a straight line from the sun to the object being heated, the temperature of any spacecraft, satellite, or other equipment is entirely dependent on whether it’s in the sun or the shade. The temperature of components in the shade can be -100° C (-148° F) or colder and can reach 260° C (500° F) in the sun4.
The “base” temperature of space, not considering the heat added by the sun or any other source, is about 2.7 Kelvin (-270° C or -455° F), which is just slightly above absolute zero. A satellite experiences the sun shining on one side and shade on the other. Therefore an extreme temperature difference occurs from one side to the other. This presents technical challenges in regard to material strength and thermal insulation that require cutting edge solutions. In order to test these advanced technologies, space test chambers must be able to produce high and low temperatures in addition to ultra-high vacuum. Both are required to ensure space components function properly under such harsh extremes of both vacuum and temperature.
The Development of Space Chambers
With the advent of high-altitude flight in the early 1900s, the first vacuum chamber was used to simulate the reduced pressure of high altitudes. In the 1960s the space program required test chambers capable of producing a higher level of vacuum to simulate the extremely low pressure encountered in space. The Space Power Chamber No. 1 (SPC) was among the first of a wave of large vacuum chambers that emerged in the early 1960s (Fig. 3).
The Space Power Facility at NASA’s Plum Brook Station (Fig. 4), which began operation in 1969, remains the largest vacuum chamber in the world. It measures 30.5 meters (100 feet) in diameter and 37.2 meters (122 feet) tall. The door 15.2 meters wide by 15.2 meters high (50 foot by 50 foot). Originally used to test the thermal vacuum-readiness of spacecraft and rocket fairing separations, it has been upgraded to test the new Constellation class starship, Orion, and other state-of-the-art spacecraft propulsion systems. It was also employed to test the airbag landing systems for the Mars rovers.
The chamber utilizes a double wall construction incorporating a type 5083 aluminum fully welded interior, surrounded by 1.8 to 2.4 meter (6 to 8 feet) thick steel reinforced concrete walls. The chamber has the capability of cooling the interior to -196° C (-320° F), as well as simulating the radiant heat of the sun with 400 kW infrared heaters, and providing a vacuum of 10-6 mbar.
