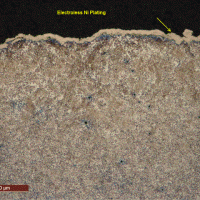
Decarburization occurs when carbon atoms at the steel surface interact with the furnace atmosphere and are removed from the steel as a gaseous phase (1-8). Carbon from the interior will then diffuse towards the surface, that is, carbon diffuses from a region of high concentration to a region of low concentration to continue the decarburization process and establish the maximum depth of decarburization (MAD).
Because the rate of carbon diffusion increases with temperature when the structure is fully austenitic, the MAD will increase as the temperature increases above the Ac3. For temperatures in the two phase region, between the Ac1 and Ac3, the process is more complex. The diffusion rates of carbon in ferrite and in austenite are different and are influenced by temperature and composition.


 Over the past forty-plus years, steelmakers have introduced improved practices for reducing the inclusion content of steels. The success of these practice changes can be monitored in a variety of ways. Chemical analysis of the bulk sulfur and oxygen contents provides a relatively simple means to assess the impact of these changes. However, microscopical test methods are still needed to assess the nature of the inclusions present.
Over the past forty-plus years, steelmakers have introduced improved practices for reducing the inclusion content of steels. The success of these practice changes can be monitored in a variety of ways. Chemical analysis of the bulk sulfur and oxygen contents provides a relatively simple means to assess the impact of these changes. However, microscopical test methods are still needed to assess the nature of the inclusions present.