Picric acid (2,4,6-trinitrophenol, [(NO2)3C6H2OH]) is widely used in metallography labs for the common steel etchants known as picral, a 4% solution in ethanol, Vilella’s reagent, 1 g picric acid and 5 mL HCl and 100 mL ethanol, and alkaline sodium picrate (2 g picric acid, 20 g NaOH, 100 mL water) for coloring M3C and M6C carbides, as well as several other formulations.
Picric acid was formulated by Peter Woulfe, a British chemist, in 1771, although Glauber is claimed to have written about it in 1742. The name comes from the Greek word pikros which means bitter, as picric acid has a bitter taste (it is toxic). Initially it was used to dye fabrics yellow. In the early 20th century, workers producing picric acid were sometimes called canaries, because their skin also became stained yellow.


 Numerous etchants have been used to selectively reveal matrix phases and second-phase constituents in ferritic, martensitic, ferritic-martensitic, austenitic, ferritic-austenitic (duplex) and precipitation hardenable stainless steels. Procedures for identification of second-phases, such as carbides, sigma and chi, and delta ferrite in austenitic or precipitation hardenable stainless steels using selective etchants are described.
Numerous etchants have been used to selectively reveal matrix phases and second-phase constituents in ferritic, martensitic, ferritic-martensitic, austenitic, ferritic-austenitic (duplex) and precipitation hardenable stainless steels. Procedures for identification of second-phases, such as carbides, sigma and chi, and delta ferrite in austenitic or precipitation hardenable stainless steels using selective etchants are described.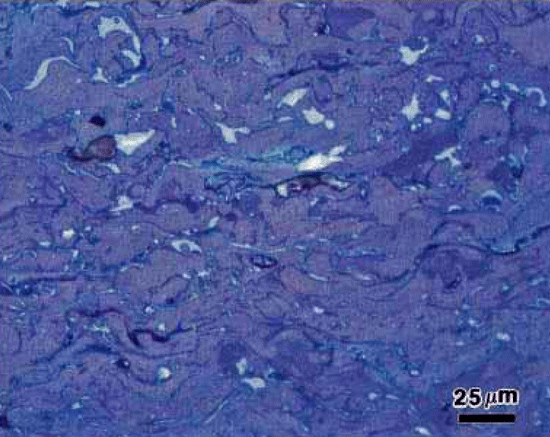
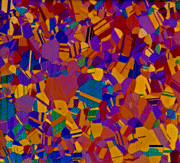
 Color has historically seen limited use in metallography, mainly due to the cost of film and prints and the difficulty and cost of reproducing images in publications. However, with the growth of digital imaging, capturing color images is much simpler and cheaper. Also, printing images in color is inexpensive for in-house reports, and can be distributed cheaply on CDs, although reproduction in journals is still expensive. Color does have many advantages over black and white. First, the human eye is sensitive to only about forty shades of gray from white to black, but is sensitive to a vast number of colors. Tint etchants reveal features in the microstructure that often cannot be revealed using standard black and white etchants. Color etchants are sensitive to crystallographic orientation and can reveal if the grains have a random or a preferred crystallographic texture. They are also very sensitive to variations in composition and residual deformation. Further, they are usually selective to certain phases and this is valuable in quantitative microscopy. By George Vander Voort
Color has historically seen limited use in metallography, mainly due to the cost of film and prints and the difficulty and cost of reproducing images in publications. However, with the growth of digital imaging, capturing color images is much simpler and cheaper. Also, printing images in color is inexpensive for in-house reports, and can be distributed cheaply on CDs, although reproduction in journals is still expensive. Color does have many advantages over black and white. First, the human eye is sensitive to only about forty shades of gray from white to black, but is sensitive to a vast number of colors. Tint etchants reveal features in the microstructure that often cannot be revealed using standard black and white etchants. Color etchants are sensitive to crystallographic orientation and can reveal if the grains have a random or a preferred crystallographic texture. They are also very sensitive to variations in composition and residual deformation. Further, they are usually selective to certain phases and this is valuable in quantitative microscopy. By George Vander Voort A wide variety of surface treatments and coatings are applied to metals to enhance their performance, for example, to improve fatigue resistance, increase wear resistance, corrosion or oxidation resistance.
A wide variety of surface treatments and coatings are applied to metals to enhance their performance, for example, to improve fatigue resistance, increase wear resistance, corrosion or oxidation resistance.